Streamlining SARS-CoV-2 confirmatory testing to reduce false positive results
- PMID: 33607351
- PMCID: PMC7877812
- DOI: 10.1016/j.jcv.2021.104762
Streamlining SARS-CoV-2 confirmatory testing to reduce false positive results
Abstract
Background: Confirmatory testing of SARS-CoV-2 results is essential to reduce false positives, but comes at a cost of significant extra workload for laboratories and increased turnaround time. A balance must be sought. We analysed our confirmatory testing pathway to produce a more refined approach in preparation for rising case numbers.
Methods: Over a 10-week low prevalence period we performed confirmatory testing on all newly positive results. Turnaround time was measured and results were analysed to identify a threshold that could be applied as a cut-off for future confirmatory testing and reduce overall workload for the laboratory.
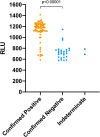
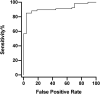
Results: Between 22/06/20 and 31/08/20 confirmatory testing was performed on 108 newly positive samples, identifying 32 false positive results (30 %). Turnaround time doubled, increasing by an extra 17 h. There was a highly statistically significant difference between initial Relative Light Unit (RLU) of results that confirmed compared to those that did not, 1176 vs 721 (P < 0.00001). RLU = 1000 was identified as a suitable threshold for confirmatory testing in our laboratory: with RLU ≥ 1000, 55/56 (98 %) confirmed as positive, whereas with RLU < 1000 only 12/38 (32 %) confirmed.
Conclusions: False positive SARS-CoV-2 tests can be identified by confirmatory testing, yet this may significantly delay results. Establishing a threshold for confirmatory testing streamlines this process to focus only on samples where it is most required. We advise all laboratories to follow a similar process to identify thresholds that trigger confirmatory testing for their own assays, increasing accuracy while maintaining efficiency for when case numbers are high.
Keywords: COVID-19; Confirmatory testing; False positive; Laboratory diagnosis; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have nothing to declare.
Figures
References
-
- GOV.UK, Public Health England, Coronavirus (COVID-19) in the UK [Internet]. Updated 14/12/2020 [cited 15/12/2020]. Available from: https://coronavirus.data.gov.uk/testing.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

