Assessing optimal: inequalities in codon optimization algorithms
- PMID: 33607980
- PMCID: PMC7893858
- DOI: 10.1186/s12915-021-00968-8
Assessing optimal: inequalities in codon optimization algorithms
Abstract
Background: Custom genes have become a common resource in recombinant biology over the last 20 years due to the plummeting cost of DNA synthesis. These genes are often "optimized" to non-native sequences for overexpression in a non-native host by substituting synonymous codons within the coding DNA sequence (CDS). A handful of studies have compared native and optimized CDSs, reporting different levels of soluble product due to the accumulation of misfolded aggregates, variable activity of enzymes, and (at least one report of) a change in substrate specificity. No study, to the best of our knowledge, has performed a practical comparison of CDSs generated from different codon optimization algorithms or reported the corresponding protein yields.
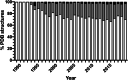
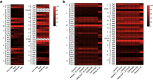
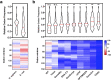
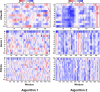
Results: In our efforts to understand what factors constitute an optimized CDS, we identified that there is little consensus among codon-optimization algorithms, a roughly equivalent chance that an algorithm-optimized CDS will increase or diminish recombinant yields as compared to the native DNA, a near ubiquitous use of a codon database that was last updated in 2007, and a high variability of output CDSs by some algorithms. We present a case study, using KRas4B, to demonstrate that a median codon frequency may be a better predictor of soluble yields than the more commonly utilized CAI metric.
Conclusions: We present a method for visualizing, analyzing, and comparing algorithm-optimized DNA sequences for recombinant protein expression. We encourage researchers to consider if DNA optimization is right for their experiments, and work towards improving the reproducibility of published recombinant work by publishing non-native CDSs.
Keywords: %MinMax; Algorithm; Codon optimization; Codon usage; KRas4B; Violin plot.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

