Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans
- PMID: 33608249
- PMCID: PMC7978427
- DOI: 10.1126/sciadv.abe8065
Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans
Abstract
Multiple preventive vaccines are being developed to counter the coronavirus disease 2019 pandemic. The leading candidates have now been evaluated in nonhuman primates (NHPs) and human phase 1 and/or phase 2 clinical trials. Several vaccines have already advanced into phase 3 efficacy trials, while others will do so before the end of 2020. Here, we summarize what is known of the antibody and T cell immunogenicity of these vaccines in NHPs and humans. To the extent possible, we compare how the vaccines have performed, taking into account the use of different assays to assess immunogenicity and inconsistencies in how the resulting data are presented. We also review the outcome of challenge experiments with severe acute respiratory syndrome coronavirus 2 in immunized macaques, while noting variations in the protocols used, including but not limited to the virus challenge doses. Press releases on the outcomes of vaccine efficacy trials are also summarized.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
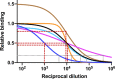

References
-
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C., Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science 369, 77–81 (2020). - PMC - PubMed
-
- Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G. F., Tan W., Yang X., Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182, 713–721.e9 (2020). - PMC - PubMed
-
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J. N., Port J. R., Avanzato V. A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E. R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Edwards N. J., Wright D., Bissett C., Gilbride C., Williamson B. N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P. W., Scott D., Saturday G., de Wit E., Gilbert S. C., Munster V. J., ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586, 578–582 (2020). - PMC - PubMed
-
- Mercado N. B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., Mahan K. M., Tostanoski L. H., He X., Martinez D. R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J. P., Kwaks T., Bakkers M. J. G., Zuijdgeest D., Rosendahl Huber S. K., Atyeo C., Fischinger S., Burke J. S., Feldman J., Hauser B. M., Caradonna T. M., Bondzie E. A., Dagotto G., Gebre M. S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S. H., Maxfield L. F., Nampanya F., Nityanandam R., Nkolola J. P., Patel S., Ventura J. D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M. G., Cai Y., Chen B., Schmidt A. G., Reeves R. K., Baric R. S., Lauffenburger D. A., Alter G., Stoffels P., Mammen M., Van Hoof J., Schuitemaker H., Barouch D. H., Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586, 583–588 (2020). - PMC - PubMed
-
- L. Solforosi, H. Kuipers, S. K. Rosendahl Huber, J. E. M. van der Lubbe, L. Dekking, D. N. Czapska-Casey, A. Izquierdo Gil, M. R. M. Baert, J. Drijver, J. Vaneman, E. van Huizen, Y. Choi, J. Vreugdenhil, T. J. Dalebout, S. K. Myeni, M. Kikkert, E. J. Snijder, D. H. Barouch, G. Koopman, P. Mooij, W. M. J. M. Bogers, L. Muchene, J. T. B. M. Tolboom, R. Roozendaal, H. Schuitemaker, F. Wegmann, R. C. Zahn, Immunogenicity of one- and two-dose regimens of the Ad26.COV2.S COVID-19 vaccine candidate in adult and aged rhesus macaques. bioRxiv 2020.11.17.368258 [Preprint]. 17 November 2020. 10.1101/2020.11.17.368258. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

