Seafloor Incubation Experiment with Deep-Sea Hydrothermal Vent Fluid Reveals Effect of Pressure and Lag Time on Autotrophic Microbial Communities
- PMID: 33608294
- PMCID: PMC8091007
- DOI: 10.1128/AEM.00078-21
Seafloor Incubation Experiment with Deep-Sea Hydrothermal Vent Fluid Reveals Effect of Pressure and Lag Time on Autotrophic Microbial Communities
Abstract
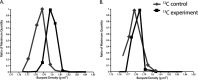
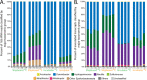
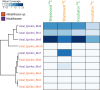
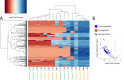
Depressurization and sample processing delays may impact the outcome of shipboard microbial incubations of samples collected from the deep sea. To address this knowledge gap, we developed a remotely operated vehicle (ROV)-powered incubator instrument to carry out and compare results from in situ and shipboard RNA stable isotope probing (RNA-SIP) experiments to identify the key chemolithoautotrophic microbes and metabolisms in diffuse, low-temperature venting fluids from Axial Seamount. All the incubations showed microbial uptake of labeled bicarbonate primarily by thermophilic autotrophic Epsilonbacteraeota that oxidized hydrogen coupled with nitrate reduction. However, the in situ seafloor incubations showed higher abundances of transcripts annotated for aerobic processes, suggesting that oxygen was lost from the hydrothermal fluid samples prior to shipboard analysis. Furthermore, transcripts for thermal stress proteins such as heat shock chaperones and proteases were significantly more abundant in the shipboard incubations, suggesting that depressurization induced thermal stress in the metabolically active microbes in these incubations. Together, the results indicate that while the autotrophic microbial communities in the shipboard and seafloor experiments behaved similarly, there were distinct differences that provide new insight into the activities of natural microbial assemblages under nearly native conditions in the ocean.IMPORTANCE Diverse microbial communities drive biogeochemical cycles in Earth's ocean, yet studying these organisms and processes is often limited by technological capabilities, especially in the deep ocean. In this study, we used a novel marine microbial incubator instrument capable of in situ experimentation to investigate microbial primary producers at deep-sea hydrothermal vents. We carried out identical stable isotope probing experiments coupled to RNA sequencing both on the seafloor and on the ship to examine thermophilic, microbial autotrophs in venting fluids from an active submarine volcano. Our results indicate that microbial communities were significantly impacted by the effects of depressurization and sample processing delays, with shipboard microbial communities being more stressed than seafloor incubations. Differences in metabolism were also apparent and are likely linked to the chemistry of the fluid at the beginning of the experiment. Microbial experimentation in the natural habitat provides new insights into understanding microbial activities in the ocean.
Keywords: RNA-SIP; autotrophy; deep sea; hydrothermal vent; instrumentation; metagenomics; metatranscriptomics.
Copyright © 2021 Fortunato et al.
Figures



Similar articles
-
Spatially distinct, temporally stable microbial populations mediate biogeochemical cycling at and below the seafloor in hydrothermal vent fluids.Environ Microbiol. 2018 Feb;20(2):769-784. doi: 10.1111/1462-2920.14011. Epub 2017 Dec 15. Environ Microbiol. 2018. PMID: 29205750
-
Metagenomic Signatures of Microbial Communities in Deep-Sea Hydrothermal Sediments of Azores Vent Fields.Microb Ecol. 2018 Aug;76(2):387-403. doi: 10.1007/s00248-018-1144-x. Epub 2018 Jan 21. Microb Ecol. 2018. PMID: 29354879
-
Coupled RNA-SIP and metatranscriptomics of active chemolithoautotrophic communities at a deep-sea hydrothermal vent.ISME J. 2016 Aug;10(8):1925-38. doi: 10.1038/ismej.2015.258. Epub 2016 Feb 12. ISME J. 2016. PMID: 26872039 Free PMC article.
-
The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally.Nat Rev Microbiol. 2019 May;17(5):271-283. doi: 10.1038/s41579-019-0160-2. Nat Rev Microbiol. 2019. PMID: 30867583 Review.
-
Temporal change in deep-sea benthic ecosystems: a review of the evidence from recent time-series studies.Adv Mar Biol. 2010;58:1-95. doi: 10.1016/B978-0-12-381015-1.00001-0. Adv Mar Biol. 2010. PMID: 20959156 Review.
Cited by
-
Non-thermodynamic factors affect competition between thermophilic chemolithoautotrophs from deep-sea hydrothermal vents.Appl Environ Microbiol. 2024 Aug 21;90(8):e0029224. doi: 10.1128/aem.00292-24. Epub 2024 Jul 16. Appl Environ Microbiol. 2024. PMID: 39012100 Free PMC article.
-
Microbial eukaryotic predation pressure and biomass at deep-sea hydrothermal vents.ISME J. 2024 Jan 8;18(1):wrae004. doi: 10.1093/ismejo/wrae004. ISME J. 2024. PMID: 38366040 Free PMC article.
-
Role of deep-sea equipment in promoting the forefront of studies on life in extreme environments.iScience. 2021 Oct 16;24(11):103299. doi: 10.1016/j.isci.2021.103299. eCollection 2021 Nov 19. iScience. 2021. PMID: 34765920 Free PMC article. Review.
-
Oceanic Crustal Fluid Single Cell Genomics Complements Metagenomic and Metatranscriptomic Surveys With Orders of Magnitude Less Sample Volume.Front Microbiol. 2022 Jan 24;12:738231. doi: 10.3389/fmicb.2021.738231. eCollection 2021. Front Microbiol. 2022. PMID: 35140689 Free PMC article.
-
Approaches to Unmask Functioning of the Uncultured Microbial Majority From Extreme Habitats on the Seafloor.Front Microbiol. 2022 Mar 29;13:845562. doi: 10.3389/fmicb.2022.845562. eCollection 2022. Front Microbiol. 2022. PMID: 35422772 Free PMC article. Review.
References
-
- Butterfield DA, Roe KK, Lilley MD, Huber JA, Baross JA, Embley RW, Massoth GJ. 2004. Mixing, reaction and microbial activity in the sub-seafloor revealed by temporal and spatial variation in diffuse flow vents at Axial Volcano, p 269–289. In Wilcock WSD, DeLong EF, Kelley DS, Baross JA, Cary SC (ed), The subseafloor biosphere at mid-ocean ridges. American Geophysical Union, Washington, DC.
-
- Perner M, Bach W, Hentscher M, Koschinsky A, Garbe-Schonberg D, Streit WR, Strauss H. 2009. Short-term microbial and physico-chemical variability in low-temperature hydrothermal fluids near 5°S on the Mid-Atlantic Ridge. Environ Microbiol 11:2526–2541. 10.1111/j.1462-2920.2009.01978.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

