Androgen Receptor and PIM1 Expression in Tumor Tissue of Patients With Triple-negative Breast Cancer
- PMID: 33608311
- PMCID: PMC7943211
- DOI: 10.21873/cgp.20249
Androgen Receptor and PIM1 Expression in Tumor Tissue of Patients With Triple-negative Breast Cancer
Abstract
Background/aim: Effective targeted therapies for triple-negative breast cancer (TNBC) are limited. In a subset of TNBC, androgen receptor (AR) plays an important role, while the human proviral integration site for Moloney murine leukemia virus-1 (PIM1) overexpression is also implicated. PIM1 kinases phosphorylate AR, thus regulating its transcriptional activity, regardless of the presence or not of androgens. We evaluated the expression of AR and PIM1 and their prognostic significance in TNBC.
Materials and methods: AR and PIM1 transcripts were quantified by quantitative reverse transcription polymerase chain reaction in formalin-fixed paraffin-embedded tumor from 141 patients with TNBC.
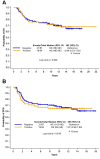
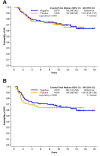
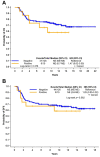
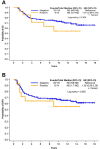
Results: AR was expressed in 38.3%, PIM1 in 10.6%, while co-expression of AR and PIM1 was detected in 7/141 cases (5.0%). No prognostic significance of AR or PIM1 was reached for overall or disease-free survival.
Conclusion: Co-expression of AR and PIM1 exists in only in a small percentage of patients with TNBC. The implications of this finding in the therapeutic management of patients with TNBC should be investigated in larger patient cohorts.
Keywords: PIM1; RT-qPCR; Triple-negative breast cancer; androgen receptor.
Copyright© 2021, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
There are no conflicts of interest to declare for all Authors in regard to this study
Figures
References
-
- Huang M, O’Shaughnessy J, Zhao J, Haiderali A, Cortés J, Ramsey SD, Briggs A, Hu P, Karantza V, Aktan G, Qi CZ, Gu C, Xie J, Yuan M, Cook J, Untch M, Schmid P, Fasching PA. Association of pathologic complete response with long-term survival outcomes in triple-negative breast cancer: A meta-analysis. Cancer Res. 2020;80(24):5427–5434. doi: 10.1158/0008-5472.CAN-20-1792. - DOI - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
