A clinical score for neuroendocrine tumor patients under consideration for Lu-177-DOTATATE therapy
- PMID: 33608484
- PMCID: PMC8026653
- DOI: 10.1530/ERC-20-0482
A clinical score for neuroendocrine tumor patients under consideration for Lu-177-DOTATATE therapy
Abstract
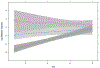
We developed a clinical score (CS) at Vanderbilt Ingram Cancer Center (VICC) that we hoped would predict outcomes for patients with progressive well-differentiated neuroendocrine tumors (NETs) receiving therapy with Lutetium-177 (177Lu)-DOTATATE. Patients under consideration for 177Lu-DOTATATE between March 1, 2016 and March 17, 2020 at VICC were assigned a CS prospectively. The CS included 5 categories: available treatments for tumor type outside of 177Lu-DOTATATE, prior systemic treatments, patient symptoms, tumor burden in critical organs and presence of peritoneal carcinomatosis. The primary outcome of the analysis was progression-free survival (PFS). To evaluate the effect of the CS on PFS, a multivariable Cox regression analysis was performed adjusting for tumor grade, primary tumor location, and the interaction between 177Lu-DOTATATE doses received (zero, 1-2, 3-4) and CS. A total of 91 patients and 31 patients received 3-4 doses and zero doses of 177Lu-DOTATATE, respectively. On multivariable analysis, in patients treated with 3-4 doses of 177Lu-DOTATATE, for each 1-point increase in CS, the estimated hazard ratio (HR) for PFS was 2.0 (95% CI 1.61-2.48). On multivariable analysis, in patients who received zero doses of 177Lu-DOTATATE, for each 1-point increase in CS, the estimated HR for PFS was 1.22 (95% CI 0.91-1.65). Among patients treated with 3-4 doses of 177Lu-DOTATATE, those with lower CS experienced improved PFS with the treatment compared to patients with higher CS. This PFS difference, based upon CS, was not observed in patients who did not receive 177Lu-DOTATATE, suggesting the predictive utility of the score.
Keywords: clinical score; lutetium-177-DOTATATE; patient outcomes; peptide receptor radionuclide therapy.
Conflict of interest statement
Declaration of Interest
None of the authors declare a conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Ballal S, Yadav M, Bal C, Sahoo R & Tripathi M 2019. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. European Journal of Nuclear Medicine and Molecular Imaging 47 943–946. - PubMed
-
- Baum R, Kulkarni H, Singh A, Kaemmerer D, Mueller D, Prasad V, Homman M, Robiller F, Niepsch K, Franz H et al. 2018. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 9 16932–16950. - PMC - PubMed
-
- Bodei L, Kidd M, Singh A, Drozdov I, Malczewska A, Baum R, Krenning E & Modlin I 2020. The utility of blood-based molecular tools-the NETest-to monitor and evaluate the efficacy of PRRT in neuroendocrine tumors. Journal of Clinical Oncology 38 3568–3568.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

