Diatom fucan polysaccharide precipitates carbon during algal blooms
- PMID: 33608542
- PMCID: PMC7896085
- DOI: 10.1038/s41467-021-21009-6
Diatom fucan polysaccharide precipitates carbon during algal blooms
Abstract
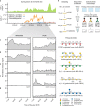
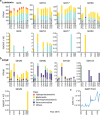
The formation of sinking particles in the ocean, which promote carbon sequestration into deeper water and sediments, involves algal polysaccharides acting as an adhesive, binding together molecules, cells and minerals. These as yet unidentified adhesive polysaccharides must resist degradation by bacterial enzymes or else they dissolve and particles disassemble before exporting carbon. Here, using monoclonal antibodies as analytical tools, we trace the abundance of 27 polysaccharide epitopes in dissolved and particulate organic matter during a series of diatom blooms in the North Sea, and discover a fucose-containing sulphated polysaccharide (FCSP) that resists enzymatic degradation, accumulates and aggregates. Previously only known as a macroalgal polysaccharide, we find FCSP to be secreted by several globally abundant diatom species including the genera Chaetoceros and Thalassiosira. These findings provide evidence for a novel polysaccharide candidate to contribute to carbon sequestration in the ocean.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Alldredge AL, Gotschalk C, Passow U, Riebesell U. Mass aggregation of diatom blooms: insights from a mesocosm study. Deep Sea Res. Part II Top. Stud. Oceanogr. 1995;42:9–27. doi: 10.1016/0967-0645(95)00002-8. - DOI
-
- Eppley RW, Peterson BJ. Particulate organic matter flux and planktonic new production in the deep ocean. Nature. 1979;282:677–680. doi: 10.1038/282677a0. - DOI
-
- Alldredge AL, Gotschalk CC. Direct observations of the mass flocculation of diatom blooms: characteristics, settling velocities and formation of diatom aggregates. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1989;36:159–171. doi: 10.1016/0198-0149(89)90131-3. - DOI
-
- Le Quéré C, et al. Global carbon budget 2018. Earth Syst. Sci. Data. 2018;10:2141–2194. doi: 10.5194/essd-10-2141-2018. - DOI
-
- Aluwihare LI, Repeta DJ, Chen RF. A major biopolymeric component to dissolved organic carbon in surface sea water. Nature. 1997;387:166–169. doi: 10.1038/387166a0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

