Calculating metalation in cells reveals CobW acquires CoII for vitamin B12 biosynthesis while related proteins prefer ZnII
- PMID: 33608553
- PMCID: PMC7895991
- DOI: 10.1038/s41467-021-21479-8
Calculating metalation in cells reveals CobW acquires CoII for vitamin B12 biosynthesis while related proteins prefer ZnII
Abstract
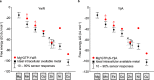
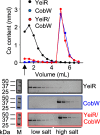
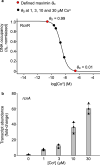
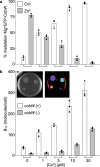
Protein metal-occupancy (metalation) in vivo has been elusive. To address this challenge, the available free energies of metals have recently been determined from the responses of metal sensors. Here, we use these free energy values to develop a metalation-calculator which accounts for inter-metal competition and changing metal-availabilities inside cells. We use the calculator to understand the function and mechanism of GTPase CobW, a predicted CoII-chaperone for vitamin B12. Upon binding nucleotide (GTP) and MgII, CobW assembles a high-affinity site that can obtain CoII or ZnII from the intracellular milieu. In idealised cells with sensors at the mid-points of their responses, competition within the cytosol enables CoII to outcompete ZnII for binding CobW. Thus, CoII is the cognate metal. However, after growth in different [CoII], CoII-occupancy ranges from 10 to 97% which matches CobW-dependent B12 synthesis. The calculator also reveals that related GTPases with comparable ZnII affinities to CobW, preferentially acquire ZnII due to their relatively weaker CoII affinities. The calculator is made available here for use with other proteins.
Conflict of interest statement
The authors declare no competing interests.
Figures
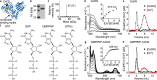
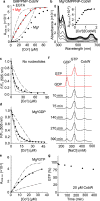
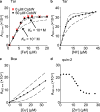
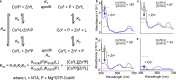

References
-
- Irving H, Williams RJP. Order of stability of metal complexes. Nature. 1948;162:746–747. doi: 10.1038/162746a0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- BB/S014020/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/S009787/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/T019891/1/MRC_/Medical Research Council/United Kingdom
- BB/L013711/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J017787/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

