Cellular and functional heterogeneity of the airway epithelium
- PMID: 33608655
- PMCID: PMC7893625
- DOI: 10.1038/s41385-020-00370-7
Cellular and functional heterogeneity of the airway epithelium
Erratum in
-
Correction: Cellular and functional heterogeneity of the airway epithelium.Mucosal Immunol. 2022 Mar;15(3):528. doi: 10.1038/s41385-022-00500-3. Mucosal Immunol. 2022. PMID: 35296789 Free PMC article. No abstract available.
Abstract
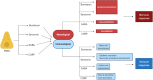
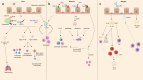
The airway epithelium protects us from environmental insults, which we encounter with every breath. Not only does it passively filter large particles, it also senses potential danger and alerts other cells, including immune and nervous cells. Together, these tissues orchestrate the most appropriate response, balancing the need to eliminate the danger with the risk of damage to the host. Each cell subset within the airway epithelium plays its part, and when impaired, may contribute to the development of respiratory disease. Here we highlight recent advances regarding the cellular and functional heterogeneity along the airway epithelium and discuss how we can use this knowledge to design more effective, targeted therapeutics.
© 2021. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Karra N, Swindle EJ, Morgan H. Drug delivery for traditional and emerging airway models. Organs-on-a-Chip. 2019;1:100002. doi: 10.1016/j.ooc.2020.100002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

