Development and validation of a reinforcement learning algorithm to dynamically optimize mechanical ventilation in critical care
- PMID: 33608661
- PMCID: PMC7895944
- DOI: 10.1038/s41746-021-00388-6
Development and validation of a reinforcement learning algorithm to dynamically optimize mechanical ventilation in critical care
Abstract
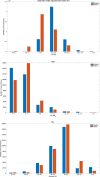
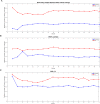
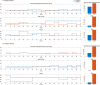
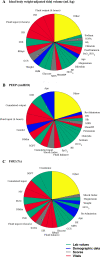
The aim of this work was to develop and evaluate the reinforcement learning algorithm VentAI, which is able to suggest a dynamically optimized mechanical ventilation regime for critically-ill patients. We built, validated and tested its performance on 11,943 events of volume-controlled mechanical ventilation derived from 61,532 distinct ICU admissions and tested it on an independent, secondary dataset (200,859 ICU stays; 25,086 mechanical ventilation events). A patient "data fingerprint" of 44 features was extracted as multidimensional time series in 4-hour time steps. We used a Markov decision process, including a reward system and a Q-learning approach, to find the optimized settings for positive end-expiratory pressure (PEEP), fraction of inspired oxygen (FiO2) and ideal body weight-adjusted tidal volume (Vt). The observed outcome was in-hospital or 90-day mortality. VentAI reached a significantly increased estimated performance return of 83.3 (primary dataset) and 84.1 (secondary dataset) compared to physicians' standard clinical care (51.1). The number of recommended action changes per mechanically ventilated patient constantly exceeded those of the clinicians. VentAI chose 202.9% more frequently ventilation regimes with lower Vt (5-7.5 mL/kg), but 50.8% less for regimes with higher Vt (7.5-10 mL/kg). VentAI recommended 29.3% more frequently PEEP levels of 5-7 cm H2O and 53.6% more frequently PEEP levels of 7-9 cmH2O. VentAI avoided high (>55%) FiO2 values (59.8% decrease), while preferring the range of 50-55% (140.3% increase). In conclusion, VentAI provides reproducible high performance by dynamically choosing an optimized, individualized ventilation strategy and thus might be of benefit for critically ill patients.
Conflict of interest statement
A.P., G.D., A.S., C.T., G.M., and L.M. are co-founders of Clinomic GmbH. A.P. and L.M. are chief executive officers of Clinomic GmbH. C.T. is chief executive officer of William Harvey Research Limited outside of the submitted work. G.M. received restricted research grants and consultancy fees from BBraun Melsungen, Biotest, Adrenomed, and Sphingotec GmbH outside of the submitted work. L.M. and A.P. received consultancy fees from Sphingotec GmbH. All remaining authors declare that they have no conflict of interests.
Figures


References
Grants and funding
- R01 EB017205/EB/NIBIB NIH HHS/United States
- 13GW0280D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- EIT-Health 19549/EC | EU Framework Programme for Research and Innovation H2020 | H2020 European Institute of Innovation and Technology (H2020 The European Institute of Innovation and Technology)
- 13GW0280E/Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Federal Ministry for Education, Science, Research and Technology)
- 13GW0280C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Other Literature Sources

