Simvastatin Augmentation for Patients With Early-Phase Schizophrenia-Spectrum Disorders: A Double-Blind, Randomized Placebo-Controlled Trial
- PMID: 33608711
- PMCID: PMC8266622
- DOI: 10.1093/schbul/sbab010
Simvastatin Augmentation for Patients With Early-Phase Schizophrenia-Spectrum Disorders: A Double-Blind, Randomized Placebo-Controlled Trial
Abstract
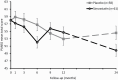
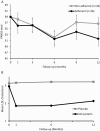
Schizophrenia-spectrum disorders (SSD) are associated with increased inflammatory markers, both in brain and periphery. Augmentation with drugs that lower this pro-inflammatory status may improve clinical presentation. Simvastatin crosses the blood-brain barrier, has anti- inflammatory and neuroprotective effects and reduces metabolic syndrome. In this study, we investigated if 12 months of simvastatin augmentation can improve symptoms and cognition in patients with early SSD. This double-blind placebo-controlled trial included 127 SSD patients across the Netherlands, <3 years after their diagnosis. From these, 119 were randomly assigned 1:1 to simvastatin 40 mg (n = 61) or placebo (n = 58), stratified for sex and study site. Primary outcomes were symptom severity and cognition after 12 months of treatment. Depression, symptom subscores, general functioning, metabolic syndrome, movement disorders, and safety were secondary outcomes. Intention to treat analyses were performed using linear mixed models and ANCOVA. No main effect of simvastatin treatment was found on total symptom severity after 12 months of treatment as compared to placebo (X2(1) = 0.01, P = .90). Group differences varied over time (treatment*time X2(4) = 11.2; P = .025), with significantly lower symptom severity in the simvastatin group after 6 months (mean difference = -4.8; P = .021; 95% CI: -8.8 to -0.7) and at 24 months follow-up (mean difference = -4.7; P = .040; 95% CI: -9.3 to -0.2). No main treatment effect was found for cognition (F(1,0.1) = 0.37, P = .55) or secondary outcomes. SAEs occurred more frequently with placebo (19%) than with simvastatin (6.6%). This negative finding corroborates other large scale studies on aspirin, minocycline, and celecoxib that could not replicate positive findings of smaller studies, and suggests that anti-inflammatory augmentation does not improve the clinical presentation of SSD.
Keywords: RCT; inflammation; schizophrenia; simvastatin; symptoms.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center.
Figures

References
-
- Levi L, Bar Haim M, Burshtein S, et al. . Duration of untreated psychosis and response to treatment: an analysis of response in the OPTiMiSE cohort. Eur Neuropsychopharmacol. 2020;32:131–135. - PubMed
-
- Bucci P, Mucci A, van Rossum IW, et al. . Persistent negative symptoms in recent-onset psychosis: relationship to treatment response and psychosocial functioning. Eur Neuropsychopharmacol. 2020;34:76–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

