Sodium-glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial
- PMID: 33609072
- PMCID: PMC8358952
- DOI: 10.1002/ejhf.2137
Sodium-glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial
Abstract
Aims: Treatment with sodium-glucose co-transporter 2 (SGLT2) inhibitors improves outcomes in patients with chronic heart failure (HF) with reduced ejection fraction. There is limited experience with the in-hospital initiation of SGLT2 inhibitors in patients with acute HF (AHF) with or without diabetes. EMPULSE is designed to assess the clinical benefit and safety of the SGLT2 inhibitor empagliflozin compared with placebo in patients hospitalized with AHF.
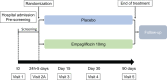
Methods: EMPULSE is a randomized, double-blind, parallel-group, placebo-controlled multinational trial comparing the in-hospital initiation of empagliflozin (10 mg once daily) with placebo. Approximately 500 patients admitted for AHF with dyspnoea, signs of fluid overload, and elevated natriuretic peptides will be randomized 1:1 stratified to HF status (de-novo and decompensated chronic HF) to either empagliflozin or placebo at approximately 165 sites across North America, Europe and Asia. Patients will be enrolled regardless of ejection fraction and diabetes status and will be randomized during hospitalization and after stabilization (between 24 h and 5 days after admission), with treatment continued up to 90 days after initiation. The primary outcome is clinical benefit at 90 days, consisting of a composite of all-cause death, HF events, and ≥5 point change from baseline in Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ-TSS), assessed using a 'win-ratio' approach. Secondary outcomes include assessments of safety, change in KCCQ-TSS from baseline to 90 days and change in natriuretic peptides from baseline to 30 days.
Conclusion: The EMPULSE trial will evaluate the clinical benefit and safety of empagliflozin in patients hospitalized for AHF.
Keywords: Heart failure; Sodium-glucose co-transporter 2 inhibitors; Trial design.
© 2021 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
References
-
- Tromp J, Ferreira JP, Janwanishstaporn S, Shah M, Greenberg B, Zannad F, Lam CS. Heart failure around the world. Eur J Heart Fail 2019;21:1187–1196. - PubMed
-
- Tromp J, Bamadhaj S, Cleland JG, Angermann CE, Dahlstrom U, Ouwerkerk W, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Lam CS, Filippatos G, Collins SP. Post‐discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT‐HF): a cohort study. Lancet Glob Health 2020;8:e411–e422. - PubMed
-
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. - PMC - PubMed
-
- Voors AA, Van Veldhuisen DJ. Why do drugs for acute heart failure fail? Eur J Heart Fail 2012;14:955–956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous