Combined APRI/ALBI score to predict mortality after hepatic resection
- PMID: 33609383
- PMCID: PMC7893465
- DOI: 10.1093/bjsopen/zraa043
Combined APRI/ALBI score to predict mortality after hepatic resection
Abstract
Background: Aspartate aminotransferase/platelet ratio index (APRI) and albumin-bilirubin grade (ALBI) are validated prognostic indices implicated as predictors of postoperative liver dysfunction after hepatic resection. The aim of this study was to evaluate the relevance of the combined APRI/ALBI score for postoperative clinically meaningful outcomes.
Methods: Patients undergoing hepatectomy were included from the American College of Surgeons National Surgical Quality Improvement Program database. The association between APRI/ALBI score and postoperative grade C liver dysfunction, liver dysfunction-associated and overall 30-day mortality was assessed.
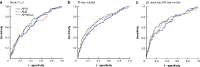
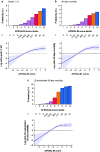
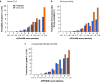
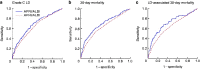
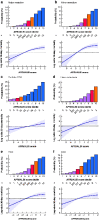
Results: A total of 12 055 patients undergoing hepatic resection from 2014 to 2017 with preoperative blood values and detailed 30-day postoperative outcomes were included (exploration cohort: January 2014 to December 2016; validation cohort: 2017). In the exploration cohort (8538 patients), the combination of both scores (APRI/ALBI) was significantly associated with postoperative grade C liver dysfunction, 30-day mortality, and liver dysfunction-associated 30-day mortality, and was superior to either score alone. The association with postoperative 30-day mortality was confirmed in multivariable analysis. A predictive model was generated using the exploration cohort. The predicted incidence of events closely followed the observed incidence in the validation cohort (3517 patients). Subgroup analyses of tumour types were used to generate disease-specific risk models to assess risk in different clinical scenarios. These findings informed development of a smartphone application (https://tellaprialbi.37binary.com).
Conclusion: The predictive potential of the combined APRI/ALBI score for clinically relevant outcomes such as mortality was demonstrated. An evidence-based smartphone application will allow clinical translation and facilitation of risk assessment before hepatic resection using routine laboratory parameters.
Antecedentes: El índice de relación aspartato aminotransferasa/plaquetas (aspartate aminotransferase/platelet ratio index, APRI) y grado de albúmina-bilirrubina (albumin-bilirubin grade, ALBI) son índices pronóstico validados implicados como predictores de disfunción hepática postoperatoria (liver dysfunction, LD) después de una resección hepática. El objetivo de este estudio fue evaluar la relevancia de la puntuación combinada APRI/ALBI respecto los resultados postoperatorios clínicamente significativos.
Métodos:
: Los pacientes sometidos a hepatectomía se incluyeron en la base de datos del American College of Surgeons National Surgical Quality Improvement Program (NSQIP). Se evaluó la asociación de APRI/LBI con LD grado C postoperatorio, así como con LD asociada y mortalidad global a los 30 días.
Resultados:
:
Se incluyeron 12.055 pacientes sometidos a resección hepática entre 2014 y 2017 con valores sanguíneos preoperatorios y resultados postoperatorios detallados a los 30 días (cohorte de exploración: 01/2014-12/2016; cohorte de validación: 2017). En la cohorte de exploración (n = 8.538), la combinación de ambas puntuaciones (APRI/ALBI) se asoció significativamente con LD grado C postoperatorio, mortalidad a 30 días, mortalidad asociada a LD a los 30 días y fue superior a cualquiera de las puntuaciones por separado. La asociación con la mortalidad postoperatoria a los 30 días se confirmó mediante el análisis multivariable. Se generó un modelo predictivo utilizando la cohorte de exploración. La incidencia anticipada de eventos se ajustó a la incidencia observada en la cohorte de validación (n = 3.517). Se utilizaron análisis de subgrupos de tipos de tumores a fin de generar modelos de riesgo específicos de la enfermedad para evaluar el riesgo en diferentes escenarios clínicos. Estos hallazgos permitieron el desarrollo de una aplicación para teléfonos inteligentes (
Conclusión:
: Se demostró el potencial predictivo de la puntuación combinada APRI/ALBI para parámetros de resultados clínicamente relevantes, tales como la mortalidad. Una aplicación de teléfono inteligente basada en la evidencia permite la traducción clínica y facilita la evaluación de riesgos antes de la resección hepática utilizando parámetros de laboratorio de rutina.
© The Author(s) 2021. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures

References
-
- Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol 2015;26:702–708 - PubMed
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007–1016 - PMC - PubMed
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–1215 - PubMed
-
- Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274–286 - PubMed
-
- Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065–2072 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

