Efficient inhibition of O-glycan biosynthesis using the hexosamine analog Ac5GalNTGc
- PMID: 33609441
- PMCID: PMC8140985
- DOI: 10.1016/j.chembiol.2021.01.017
Efficient inhibition of O-glycan biosynthesis using the hexosamine analog Ac5GalNTGc
Abstract
There is a critical need to develop small-molecule inhibitors of mucin-type O-linked glycosylation. The best-known reagent currently is benzyl-GalNAc, but it is effective only at millimolar concentrations. This article demonstrates that Ac5GalNTGc, a peracetylated C-2 sulfhydryl-substituted GalNAc, fulfills this unmet need. When added to cultured leukocytes, breast cells, and prostate cells, Ac5GalNTGc increased cell-surface VVA binding by ∼10-fold, indicating truncation of O-glycan biosynthesis. Cytometry, mass spectrometry, and western blot analysis of HL-60 promyelocytes demonstrated that 50-80 μM Ac5GalNTGc prevented elaboration of 30%-60% of the O-glycans beyond the Tn-antigen (GalNAcα1-Ser/Thr) stage. The effect of the compound on N-glycans and glycosphingolipids was small. Glycan inhibition induced by Ac5GalNTGc resulted in 50%-80% reduction in leukocyte sialyl-Lewis X expression and L-/P-selectin-mediated rolling under flow conditions. Ac5GalNTGc was pharmacologically active in mouse. It reduced neutrophil infiltration to sites of inflammation by ∼60%. Overall, Ac5GalNTGc may find diverse applications as a potent inhibitor of O-glycosylation.
Keywords: O-glycan; cell adhesion; glycosylation; inflammation; inhibitor; mucin; neutrophil; selectin; sialyl-Lewis X; small molecule.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We do not have competing financial or non-financial interests.
Figures
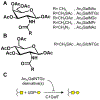




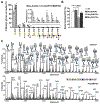

References
-
- Agarwal K, Kaul R, Garg M, Shajahan A, Jha SK, and Sampathkumar SG (2013). Inhibition of mucin-type O-glycosylation through metabolic processing and incorporation of N-thioglycolyl-D-galactosamine peracetate (Ac5GalNTGc). J Am Chem Soc 135, 14189–14197. - PubMed
-
- Alfalah M, Jacob R, Preuss U, Zimmer KP, Naim H, and Naim HY (1999). O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr Biol 9, 593–596. - PubMed
-
- Barthel SR, Antonopoulos A, Cedeno-Laurent F, Schaffer L, Hernandez G, Patil SA, North SJ, Dell A, Matta KL, Neelamegham S, et al. (2011). Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J Biol Chem 286, 21717–21731. - PMC - PubMed
-
- Beauharnois ME, Lindquist KC, Marathe D, Vanderslice P, Xia J, Matta KL, and Neelamegham S (2005). Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry 44, 9507–9519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

