Invisible leashes: The tethering VAPs from infectious diseases to neurodegeneration
- PMID: 33609524
- PMCID: PMC8005810
- DOI: 10.1016/j.jbc.2021.100421
Invisible leashes: The tethering VAPs from infectious diseases to neurodegeneration
Abstract
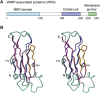
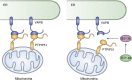
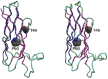
Intracellular organelles do not, as thought for a long time, act in isolation but are dynamically tethered together by entire machines responsible for interorganelle trafficking and positioning. Among the proteins responsible for tethering is the family of VAMP-associated proteins (VAPs) that appear in all eukaryotes and are localized primarily in the endoplasmic reticulum. The major functional role of VAPs is to tether the endoplasmic reticulum with different organelles and regulate lipid metabolism and transport. VAPs have gained increasing attention because of their role in human pathology where they contribute to infections by viruses and bacteria and participate in neurodegeneration. In this review, we discuss the structure, evolution, and functions of VAPs, focusing more specifically on VAP-B for its relationship with amyotrophic lateral sclerosis and other neurodegenerative diseases.
Keywords: ALS; VAMP; endoplasmic reticulum; tethering proteins.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins.Molecules. 2025 Mar 8;30(6):1220. doi: 10.3390/molecules30061220. Molecules. 2025. PMID: 40141996 Free PMC article. Review.
-
VAMP associated proteins are required for autophagic and lysosomal degradation by promoting a PtdIns4P-mediated endosomal pathway.Autophagy. 2019 Jul;15(7):1214-1233. doi: 10.1080/15548627.2019.1580103. Epub 2019 Feb 20. Autophagy. 2019. PMID: 30741620 Free PMC article.
-
The Interactome of the VAP Family of Proteins: An Overview.Cells. 2021 Jul 14;10(7):1780. doi: 10.3390/cells10071780. Cells. 2021. PMID: 34359948 Free PMC article. Review.
-
Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates.J Neurosci. 2007 Sep 5;27(36):9801-15. doi: 10.1523/JNEUROSCI.2661-07.2007. J Neurosci. 2007. PMID: 17804640 Free PMC article.
-
The ER Contact Proteins VAPA/B Interact with Multiple Autophagy Proteins to Modulate Autophagosome Biogenesis.Curr Biol. 2018 Apr 23;28(8):1234-1245.e4. doi: 10.1016/j.cub.2018.03.002. Epub 2018 Apr 5. Curr Biol. 2018. PMID: 29628370
Cited by
-
Spinal cord neurone loss and foot placement changes in a rat knock-in model of amyotrophic lateral sclerosis Type 8.Brain Commun. 2024 May 24;6(3):fcae184. doi: 10.1093/braincomms/fcae184. eCollection 2024. Brain Commun. 2024. PMID: 38846532 Free PMC article.
-
A guide to selecting high-performing antibodies for VAPB (UniProt ID: O95292) for use in western blot, immunoprecipitation, and immunofluorescence.F1000Res. 2024 Dec 24;13:1559. doi: 10.12688/f1000research.160226.1. eCollection 2024. F1000Res. 2024. PMID: 39916983 Free PMC article.
-
Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins.Molecules. 2025 Mar 8;30(6):1220. doi: 10.3390/molecules30061220. Molecules. 2025. PMID: 40141996 Free PMC article. Review.
-
In vivo proximity biotin ligation identifies the interactome of Egalitarian, a Dynein cargo adaptor.Development. 2021 Nov 15;148(22):dev199935. doi: 10.1242/dev.199935. Epub 2021 Nov 18. Development. 2021. PMID: 35020877 Free PMC article.
-
A genetic screen to uncover mechanisms underlying lipid transfer protein function at membrane contact sites.Life Sci Alliance. 2024 Mar 18;7(6):e202302525. doi: 10.26508/lsa.202302525. Print 2024 Jun. Life Sci Alliance. 2024. PMID: 38499328 Free PMC article.
References
-
- Eisenberg-Bord M., Shai N., Schuldiner M., Bohnert M. A tether is a tether is a tether: Tethering at membrane contact sites. Dev. Cell. 2016;39:395–409. - PubMed
-
- Bohnert M. Tether me, tether me not-dynamic organelle contact sites in metabolic rewiring. Dev. Cell. 2020;54:212–225. - PubMed
-
- Verma A., Berger J.R. ALS syndrome in patients with HIV-1 infection. J. Neurol. Sci. 2006;240:59–64. - PubMed
-
- Lyons J., Venna N., Cho T.A. Atypical nervous system manifestations of HIV. Semin. Neurol. 2011;31:254–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

