Moringa oleifera leaf extracts protect BMSC osteogenic induction following peroxidative damage by activating the PI3K/Akt/Foxo1 pathway
- PMID: 33610167
- PMCID: PMC7896384
- DOI: 10.1186/s13018-021-02284-x
Moringa oleifera leaf extracts protect BMSC osteogenic induction following peroxidative damage by activating the PI3K/Akt/Foxo1 pathway
Abstract
Objective: We aimed to investigate the therapeutic effects of Moringa oleifera leaf extracts on osteogenic induction of rat bone marrow mesenchymal stem cells (BMSCs) following peroxidative damage and to explore the underlying mechanisms.
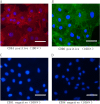
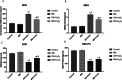
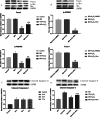
Methods: Conditioned medium was used to induce osteogenic differentiation of BMSCs, which were treated with H2O2, Moringa oleifera leaf extracts-containing serum, or the phosphatidyl inositol-3 kinase (PI3K) inhibitor wortmannin, alone or in combination. Cell viability was measured using the MTT assay. Cell cycle was assayed using flow cytometry. Expression levels of Akt, phosphorylated (p)Akt, Foxo1, and cleaved caspase-3 were analyzed using western blot analysis. The mRNA levels of osteogenesis-associated genes, including alkaline phosphatase (ALP), collagen І, osteopontin (OPN), and Runx2, were detected using qRT-PCR. Reactive oxygen species (ROS) and malondialdehyde (MDA) levels, as well as superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and ALP activity were detected using commercially available kits. Osteogenic differentiation capability was determined using alizarin red staining.
Results: During osteogenic induction of rat BMSCs, H2O2 reduced cell viability and proliferation, inhibited osteogenesis, increased ROS and MDA levels, and decreased SOD and GSH-PX activity. H2O2 significantly reduced pAkt and Foxo1 expression, and increased cleaved caspase-3 levels in BMSCs. Additional treatments with Moringa oleifera leaf extracts partially reversed the H2O2-induced changes. Wortmannin partially attenuated the effects of Moringa oleifera leaf extracts on protein expression of Foxo1, pAkt, and cleaved caspase-3, as well as mRNA levels of osteogenesis-associated genes.
Conclusion: Moringa oleifera leaf extracts ameliorate peroxidative damage and enhance osteogenic induction of rat BMSCs by activating the PI3K/Akt/Foxo1 pathway.
Keywords: Bone marrow mesenchymal stem cells; Foxo1; Hydrogen peroxide; Moringa oleifera leaf extracts; Osteogenic induction; PI3K/Akt pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Exploring the Potential of Moringa oleifera in Managing Bone Loss: Insights from Preclinical Studies.Int J Med Sci. 2025 Jan 21;22(4):819-833. doi: 10.7150/ijms.103241. eCollection 2025. Int J Med Sci. 2025. PMID: 39991771 Free PMC article. Review.
-
Ebselen rescues oxidative-stress-suppressed osteogenic differentiation of bone-marrow-derived mesenchymal stem cells via an antioxidant effect and the PI3K/Akt pathway.J Trace Elem Med Biol. 2019 Sep;55:64-70. doi: 10.1016/j.jtemb.2019.06.002. Epub 2019 Jun 10. J Trace Elem Med Biol. 2019. PMID: 31345368
-
[Mechanism of ring finger protein 11 regulating Akt signaling pathway to promote osteogenic differentiation of bone marrow mesenchymal stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 Jan 15;36(1):102-110. doi: 10.7507/1002-1892.202107108. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022. PMID: 35038807 Free PMC article. Chinese.
-
Quercetin protects rat BMSCs from oxidative stress via ferroptosis.J Mol Endocrinol. 2022 Aug 24;69(3):401-413. doi: 10.1530/JME-22-0086. Print 2022 Oct 1. J Mol Endocrinol. 2022. PMID: 35900382
-
The effects of Moringa oleifera on blood glucose levels: A scoping review of the literature.Complement Ther Med. 2020 May;50:102362. doi: 10.1016/j.ctim.2020.102362. Epub 2020 Feb 28. Complement Ther Med. 2020. PMID: 32444043
Cited by
-
Salidroside promoted osteogenic differentiation of adipose-derived stromal cells through Wnt/β-catenin signaling pathway.J Orthop Surg Res. 2021 Jul 16;16(1):456. doi: 10.1186/s13018-021-02598-w. J Orthop Surg Res. 2021. PMID: 34271966 Free PMC article.
-
The effectiveness of Moringa oleifera in the preservation of periodontium after radiation therapy: An experimental animal study.Heliyon. 2024 Mar 7;10(6):e27495. doi: 10.1016/j.heliyon.2024.e27495. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38510057 Free PMC article.
-
Exploring the Potential of Moringa oleifera in Managing Bone Loss: Insights from Preclinical Studies.Int J Med Sci. 2025 Jan 21;22(4):819-833. doi: 10.7150/ijms.103241. eCollection 2025. Int J Med Sci. 2025. PMID: 39991771 Free PMC article. Review.
-
Moringa oleifera L. Nanosuspension Extract Administration Affects Heat Shock Protein-10 and -70 under Orthodontics Mechanical Force In Vivo.Eur J Dent. 2025 May;19(2):523-530. doi: 10.1055/s-0044-1791937. Epub 2025 Jan 9. Eur J Dent. 2025. PMID: 39788532 Free PMC article.
-
Advances in the study of traditional Chinese medicine affecting bone metabolism through modulation of oxidative stress.Front Pharmacol. 2023 Nov 1;14:1235854. doi: 10.3389/fphar.2023.1235854. eCollection 2023. Front Pharmacol. 2023. PMID: 38027015 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

