Amino acid-derived defense metabolites from plants: A potential source to facilitate novel antimicrobial development
- PMID: 33610552
- PMCID: PMC8024917
- DOI: 10.1016/j.jbc.2021.100438
Amino acid-derived defense metabolites from plants: A potential source to facilitate novel antimicrobial development
Abstract
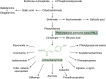
For millennia, humanity has relied on plants for its medicines, and modern pharmacology continues to reexamine and mine plant metabolites for novel compounds and to guide improvements in biological activity, bioavailability, and chemical stability. The critical problem of antibiotic resistance and increasing exposure to viral and parasitic diseases has spurred renewed interest into drug treatments for infectious diseases. In this context, an urgent revival of natural product discovery is globally underway with special attention directed toward the numerous and chemically diverse plant defensive compounds such as phytoalexins and phytoanticipins that combat herbivores, microbial pathogens, or competing plants. Moreover, advancements in "omics," chemistry, and heterologous expression systems have facilitated the purification and characterization of plant metabolites and the identification of possible therapeutic targets. In this review, we describe several important amino acid-derived classes of plant defensive compounds, including antimicrobial peptides (e.g., defensins, thionins, and knottins), alkaloids, nonproteogenic amino acids, and phenylpropanoids as potential drug leads, examining their mechanisms of action, therapeutic targets, and structure-function relationships. Given their potent antibacterial, antifungal, antiparasitic, and antiviral properties, which can be superior to existing drugs, phytoalexins and phytoanticipins are an excellent resource to facilitate the rational design and development of antimicrobial drugs.
Keywords: amino acids; antibiotic resistance; plant defense; plants; secondary metabolites.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Palumbi S.R. Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. - PubMed
-
- Coates A., Hu Y., Bax R., Page C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002;1:895–910. - PubMed
-
- Thornsberry C., Sahm D.F., Kelly L.J., Critchley I.A., Jones M.E., Evangelista A.T., Karlowsky J.A. Regional trends in antimicrobial resistance among clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: Results from the TRUST Surveillance Program, 1999-2000. Clin. Infect. Dis. 2002;34(Suppl 1):S4–S16. - PubMed
-
- Walsh C. ASM Press; Washington, DC: 2003. Antibiotics: Actions, Origins, Resistance.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

