Brazilin Removes Toxic Alpha-Synuclein and Seeding Competent Assemblies from Parkinson Brain by Altering Conformational Equilibrium
- PMID: 33610557
- PMCID: PMC7610480
- DOI: 10.1016/j.jmb.2021.166878
Brazilin Removes Toxic Alpha-Synuclein and Seeding Competent Assemblies from Parkinson Brain by Altering Conformational Equilibrium
Abstract
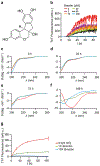
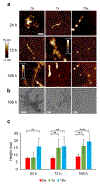
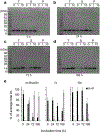
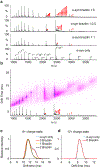
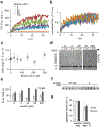
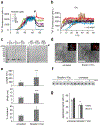
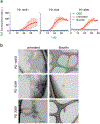
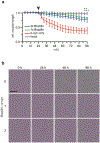
Alpha-synuclein (α-syn) fibrils, a major constituent of the neurotoxic Lewy Bodies in Parkinson's disease, form via nucleation dependent polymerization and can replicate by a seeding mechanism. Brazilin, a small molecule derived from red cedarwood trees in Brazil, has been shown to inhibit the fibrillogenesis of amyloid-beta (Aβ) and α-syn as well as remodel mature fibrils and reduce cytotoxicity. Here we test the effects of Brazilin on both seeded and unseeded α-syn fibril formation and show that the natural polyphenol inhibits fibrillogenesis of α-syn by a unique mechanism that alters conformational equilibria in two separate points of the assembly mechanism: Brazilin preserves the natively unfolded state of α-syn by specifically binding to the compact conformation of the α-syn monomer. Brazilin also eliminates seeding competence of α-syn assemblies from Parkinson's disease patient brain tissue, and reduces toxicity of pre-formed assemblies in primary neurons by inducing the formation of large fibril clusters. Molecular docking of Brazilin shows the molecule to interact both with unfolded α-syn monomers and with the cross-β sheet structure of α-syn fibrils. Our findings suggest that Brazilin has substantial potential as a neuroprotective and therapeutic agent for Parkinson's disease.
Keywords: Amyloid; Molecular Modelling; Neurdegeneration; Parkinson’s disease; Polyphenol.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Exploring Structural Insights of Aβ42 and α-Synuclein Monomers and Heterodimer: A Comparative Study Using Implicit and Explicit Solvent Simulations.J Phys Chem B. 2024 May 16;128(19):4655-4669. doi: 10.1021/acs.jpcb.4c00503. Epub 2024 May 3. J Phys Chem B. 2024. PMID: 38700150 Free PMC article.
-
Hippocampal neuronal cells that accumulate α-synuclein fragments are more vulnerable to Aβ oligomer toxicity via mGluR5--implications for dementia with Lewy bodies.Mol Neurodegener. 2014 May 19;9:18. doi: 10.1186/1750-1326-9-18. Mol Neurodegener. 2014. Retraction in: Mol Neurodegener. 2025 Aug 5;20(1):87. doi: 10.1186/s13024-025-00881-6. PMID: 24885390 Free PMC article. Retracted.
-
Effect of hydrogen sulfide on alpha-synuclein aggregation and cell viability.Sci Rep. 2025 May 4;15(1):15597. doi: 10.1038/s41598-025-99794-z. Sci Rep. 2025. PMID: 40320462 Free PMC article.
-
Unravelling the Proteinopathic Engagement of α-Synuclein, Tau, and Amyloid Beta in Parkinson's Disease: Mitochondrial Collapse as a Pivotal Driver of Neurodegeneration.Neurochem Res. 2025 Apr 16;50(3):145. doi: 10.1007/s11064-025-04399-7. Neurochem Res. 2025. PMID: 40240583 Review.
-
LRP1 at the crossroads of Parkinson's and Alzheimer's: Divergent roles in α-synuclein and amyloid pathology.Eur J Pharmacol. 2025 Sep 5;1002:177830. doi: 10.1016/j.ejphar.2025.177830. Epub 2025 Jun 6. Eur J Pharmacol. 2025. PMID: 40484331 Review.
Cited by
-
Tetracyclic homoisoflavanoid (+)-brazilin: a natural product inhibits c-di-AMP-producing enzyme and Streptococcus mutans biofilms.Microbiol Spectr. 2024 May 2;12(5):e0241823. doi: 10.1128/spectrum.02418-23. Epub 2024 Apr 9. Microbiol Spectr. 2024. PMID: 38591917 Free PMC article.
-
VCP suppresses proteopathic seeding in neurons.Mol Neurodegener. 2022 Apr 12;17(1):30. doi: 10.1186/s13024-022-00532-0. Mol Neurodegener. 2022. PMID: 35414105 Free PMC article.
-
Plant-Based Inhibitors of Protein Aggregation.Biomolecules. 2025 Mar 25;15(4):481. doi: 10.3390/biom15040481. Biomolecules. 2025. PMID: 40305223 Free PMC article. Review.
-
Controlling amyloid formation of intrinsically disordered proteins and peptides: slowing down or speeding up?Essays Biochem. 2022 Dec 16;66(7):959-975. doi: 10.1042/EBC20220046. Essays Biochem. 2022. PMID: 35975807 Free PMC article.
-
Modulation of cytotoxic amyloid fibrillation and mitochondrial damage of α-synuclein by catechols mediated conformational changes.Sci Rep. 2023 Mar 31;13(1):5275. doi: 10.1038/s41598-023-32075-9. Sci Rep. 2023. PMID: 37002248 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

