Chronic opioid use and complication risks in women with endometriosis: A cohort study in US administrative claims
- PMID: 33611812
- PMCID: PMC8251707
- DOI: 10.1002/pds.5209
Chronic opioid use and complication risks in women with endometriosis: A cohort study in US administrative claims
Abstract
Background: Women with endometriosis are prescribed opioids for pain relief but may be vulnerable to chronic opioid use given their comorbidity profile.
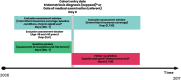
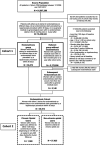
Methods: A cohort study was conducted in the Clinformatics™ DataMart database between 2006 and 2017 comparing women aged 18-50 years with endometriosis (N = 36 373) to those without (N = 2 172 936) in terms of risk of chronic opioid use, opioid dependence diagnosis, and opioid overdose. Chronic opioid use was defined as ≥120 days' supply dispensed or ≥10 fills of an opioid during any 365-day interval. Among women with endometriosis, we evaluated factors associated with higher risk of chronic opioid use and quantified the risk of complications associated with the use of opioids.
Results: Women with endometriosis were at greater risk for chronic opioid use (OR: 3.76; 95%CI: 3.57-3.96), dependence (OR: 2.73, 95%CI: 2.38-3.13) and overdose (OR: 4.34, 95%CI: 3.06-6.15) compared to women without. Chronic users displayed dose escalation and increase in days supplied over time, as well as co-prescribing with benzodiazepines and sedatives. Approximately 34% of chronic users developed constipation, 20% experienced falls, and 8% reported dizziness. Among endometriosis patients, women in younger age groups, those with other comorbidities associated with pain symptoms, as well as those with depression or anxiety were at a higher risk of developing chronic opioid use.
Conclusions: Women with endometriosis had a four times greater risk of chronic opioid use compared to women without. Multimorbidity among these patients was associated with the elevated risk of chronic opioid use and should be taken into account during treatment selection.
Keywords: chronic opioid use; chronic pain management; endometriosis-associated pain; healthcare utilization database; opioid utilization patterns.
© 2021 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
Stephanie E. Chiuve, Ryan D. Kilpatrick, Lani R. Wegrzyn, and Michael C. Snabes are employees of AbbVie receiving stock and/or stock options. Natalia Petruski‐Ivleva and Elizabeth C. Dabrowski, are employees of and holds stock at Aetion, Inc., a software and data analytics company that provides services to the healthcare industry. Priscilla Velentgas was an employee at Aetion at the time the work was completed and is currently an employee at IQVIA. Mark D. Hornstein and Brian T. Bateman conducted this work as paid consultants to Aetion, are publishing in that capacity. Mark D. Hornstein and Brian T. Bateman did not receive payment for authorship. Aetion received funding from AbbVie for conducting the study. These data were presented at the Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists, May 3–6, 2019, Nashville, TN.
Figures

References
-
- Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698‐2704. - PubMed
-
- De Graaff AA, D'Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross‐sectional survey. Hum Reprod. 2013;28:2677‐2685. - PubMed
-
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400‐412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

