Efficacy of dupilumab in patients with a history of prior sinus surgery for chronic rhinosinusitis with nasal polyps
- PMID: 33611847
- PMCID: PMC8359289
- DOI: 10.1002/alr.22780
Efficacy of dupilumab in patients with a history of prior sinus surgery for chronic rhinosinusitis with nasal polyps
Abstract
Background: Chronic rhinosinusitis with nasal polyps (CRSwNP) is a type 2 inflammatory disease treated with sinus surgery when refractory to medical intervention. However, recurrence postsurgery is common. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor for interleukin 4 (IL-4) and IL-13, key and central drivers of type 2 inflammation. We report the efficacy of dupilumab in patients with CRSwNP from the SINUS-24/SINUS-52 trials (NCT02912468/NCT02898454), by number of prior surgeries and time since last surgery.
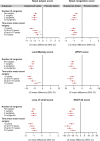
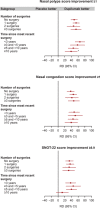
Methods: Patients were randomized to placebo or dupilumab 300 mg every 2 weeks. Post hoc subgroup analyses were performed for patients with 0, ≥1, 1/2, or ≥3 prior surgeries, and for patients who had surgery within <3, 3 to <5, 5 to <10, or ≥10 years. Efficacy outcomes at 24 weeks included co-primary endpoints nasal polyp score (NPS) and nasal congestion (NC), and Lund-Mackay (LMK), 22-item Sino-Nasal Outcome Test (SNOT-22), and smell scores.
Results: Of 724 patients randomized, 459 (63.4%) had ≥1 prior surgery. Baseline sinus disease (NPS, NC, LMK) and olfactory dysfunction (University of Pennsylvania Smell Identification Test [UPSIT] and loss of smell) scores were worse for patients with ≥3 prior surgeries vs no surgery. Baseline NPS and LMK were worse in patients with <3 years since last surgery than in patients with ≥5 years since last surgery. Dupilumab significantly improved all outcome measures vs placebo in all subgroups by number of surgeries and by time since last surgery. Improvements in NPS and LMK were greater in patients with <3 years since last surgery than patients with ≥5 years. Safety results were consistent with the known dupilumab safety profile.
Conclusion: Dupilumab improved CRSwNP outcomes irrespective of surgery history, with greater improvements in endoscopic outcomes in patients with shorter duration since last surgery.
Keywords: chronic disease; chronic rhinosinusitis; sinus surgery; subcutaneous immunotherapy; therapeutics.
© 2021 Sanofi. International Forum of Allergy & Rhinology published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngic Allergy and American Rhinologic Society.
Figures
Similar articles
-
Association Between Smell Loss, Disease Burden, and Dupilumab Efficacy in Chronic Rhinosinusitis with Nasal Polyps.Am J Rhinol Allergy. 2025 Jan;39(1):6-12. doi: 10.1177/19458924241274501. Epub 2024 Sep 19. Am J Rhinol Allergy. 2025. PMID: 39300794 Free PMC article. Clinical Trial.
-
Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials.Lancet. 2019 Nov 2;394(10209):1638-1650. doi: 10.1016/S0140-6736(19)31881-1. Epub 2019 Sep 19. Lancet. 2019. PMID: 31543428 Clinical Trial.
-
Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status.Allergy. 2022 Jan;77(1):186-196. doi: 10.1111/all.14906. Epub 2021 Jun 4. Allergy. 2022. PMID: 33993501 Free PMC article. Clinical Trial.
-
Dupilumab Versus Mepolizumab for Chronic Rhinosinusitis With Nasal Polyposis: An Indirect Treatment Comparison.J Allergy Clin Immunol Pract. 2024 Dec;12(12):3393-3401.e15. doi: 10.1016/j.jaip.2024.09.015. Epub 2024 Sep 24. J Allergy Clin Immunol Pract. 2024. PMID: 39326524
-
Comparative Effectiveness of Dupilumab Versus Sinus Surgery for Chronic Rhinosinusitis With Polyps: Systematic Review and a Meta-Analysis.Am J Rhinol Allergy. 2024 Nov;38(6):428-436. doi: 10.1177/19458924241272978. Epub 2024 Aug 16. Am J Rhinol Allergy. 2024. PMID: 39149992
Cited by
-
Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives.J Clin Med. 2025 Feb 8;14(4):1079. doi: 10.3390/jcm14041079. J Clin Med. 2025. PMID: 40004611 Free PMC article. Review.
-
Characteristics of mucin hypersecretion in different inflammatory patterns based on endotypes of chronic rhinosinusitis.Clin Transl Allergy. 2024 Jan;14(1):e12334. doi: 10.1002/clt2.12334. Clin Transl Allergy. 2024. PMID: 38282195 Free PMC article. Review.
-
Platelet-to-lymphocyte ratio might predict the response to dupilumab treatment for patients with nasal polyposis.J Otolaryngol Head Neck Surg. 2023 Nov 25;52(1):75. doi: 10.1186/s40463-023-00660-7. J Otolaryngol Head Neck Surg. 2023. PMID: 38007429 Free PMC article.
-
Type 2 Biomarkers for the Indication and Response to Biologics in CRSwNP.Curr Allergy Asthma Rep. 2023 Dec;23(12):703-713. doi: 10.1007/s11882-023-01114-w. Epub 2023 Nov 21. Curr Allergy Asthma Rep. 2023. PMID: 37987873 Review.
-
Effect of Dupilumab in CRSwNP Sinonasal Outcomes from Real Life Studies: A Systematic Review with Meta-analysis.Curr Allergy Asthma Rep. 2025 Feb 5;25(1):13. doi: 10.1007/s11882-025-01192-y. Curr Allergy Asthma Rep. 2025. PMID: 39907855 Free PMC article.
References
-
- Khan A, Vandeplas G, Huynh TMT, et al. The global allergy and asthma European network (GALEN) rhinosinusitis cohort: a large European cross‐sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32‐42. - PubMed
-
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(S29):1‐464. - PubMed
-
- Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449‐1456.e4. - PubMed
-
- Bachert C, Marple B, Hosemann W, et al. Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract. 2020;8:1514‐1519. - PubMed
-
- Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22‐S209. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

