A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair
- PMID: 33612829
- PMCID: PMC7897720
- DOI: 10.1038/s41392-020-00455-6
A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair
Abstract
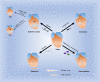
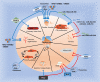
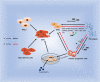
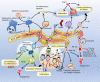
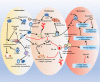
The response of immune cells in cardiac injury is divided into three continuous phases: inflammation, proliferation and maturation. The kinetics of the inflammatory and proliferation phases directly influence the tissue repair. In cardiac homeostasis, cardiac tissue resident macrophages (cTMs) phagocytose bacteria and apoptotic cells. Meanwhile, NK cells prevent the maturation and transport of inflammatory cells. After cardiac injury, cTMs phagocytose the dead cardiomyocytes (CMs), regulate the proliferation and angiogenesis of cardiac progenitor cells. NK cells prevent the cardiac fibrosis, and promote vascularization and angiogenesis. Type 1 macrophages trigger the cardioprotective responses and promote tissue fibrosis in the early stage. Reversely, type 2 macrophages promote cardiac remodeling and angiogenesis in the late stage. Circulating macrophages and neutrophils firstly lead to chronic inflammation by secreting proinflammatory cytokines, and then release anti-inflammatory cytokines and growth factors, which regulate cardiac remodeling. In this process, dendritic cells (DCs) mediate the regulation of monocyte and macrophage recruitment. Recruited eosinophils and Mast cells (MCs) release some mediators which contribute to coronary vasoconstriction, leukocyte recruitment, formation of new blood vessels, scar formation. In adaptive immunity, effector T cells, especially Th17 cells, lead to the pathogenesis of cardiac fibrosis, including the distal fibrosis and scar formation. CMs protectors, Treg cells, inhibit reduce the inflammatory response, then directly trigger the regeneration of local progenitor cell via IL-10. B cells reduce myocardial injury by preserving cardiac function during the resolution of inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

