Extracellular vesicles and pancreatitis: mechanisms, status and perspectives
- PMID: 33613112
- PMCID: PMC7893579
- DOI: 10.7150/ijbs.54858
Extracellular vesicles and pancreatitis: mechanisms, status and perspectives
Abstract
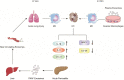
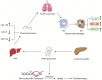
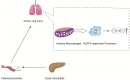
Comprehensive reviews and large population-based cohort studies have played an important role in the diagnosis and treatment of pancreatitis and its sequelae. The incidence and mortality of pancreatitis have been reduced significantly due to substantial advancements in the pathophysiological mechanisms and clinically effective treatments. The study of extracellular vesicles (EVs) has the potential to identify cell-to-cell communication in diseases such as pancreatitis. Exosomes are a subset of EVs with an average diameter of 50~150 nm. Their diverse and unique constituents include nucleic acids, proteins, and lipids, which can be transferred to trigger phenotypic changes of recipient cells. In recent years, many reports have indicated the role of EVs in pancreatitis, including acute pancreatitis, chronic pancreatitis and autoimmune pancreatitis, suggesting their potential influence on the development and progression of pancreatitis. Plasma exosomes of acute pancreatitis can effectively reach the alveolar cavity and activate alveolar macrophages to cause acute lung injury. Furthermore, upregulated exosomal miRNAs can be used as biomarkers for acute pancreatitis. Here, we summarized the current understanding of EVs in pancreatitis with an emphasis on their biological roles and their potential use as diagnostic biomarkers and therapeutic agents for this disease.
Keywords: biomarkers; exosomes; extracellular vesicles; pancreatitis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Quantitative metabolic analysis of plasma extracellular vesicles for the diagnosis of severe acute pancreatitis.J Nanobiotechnology. 2022 Jan 28;20(1):52. doi: 10.1186/s12951-022-01239-6. J Nanobiotechnology. 2022. PMID: 35090480 Free PMC article.
-
Extracellular vesicles in cardiovascular diseases: From pathophysiology to diagnosis and therapy.Cytokine Growth Factor Rev. 2023 Dec;74:40-55. doi: 10.1016/j.cytogfr.2023.09.006. Epub 2023 Sep 28. Cytokine Growth Factor Rev. 2023. PMID: 37798169 Review.
-
Intercellular communication by extracellular vesicles and their microRNAs in asthma.Clin Ther. 2014 Jun 1;36(6):873-81. doi: 10.1016/j.clinthera.2014.05.006. Epub 2014 Jun 6. Clin Ther. 2014. PMID: 24909737
-
Extracellular Vesicles in Lung Disease.Chest. 2018 Jan;153(1):210-216. doi: 10.1016/j.chest.2017.06.026. Epub 2017 Jul 3. Chest. 2018. PMID: 28684288 Review.
-
Delivery of microRNAs by Extracellular Vesicles in Viral Infections: Could the News be Packaged?Cells. 2019 Jun 18;8(6):611. doi: 10.3390/cells8060611. Cells. 2019. PMID: 31216738 Free PMC article. Review.
Cited by
-
Quantitative metabolic analysis of plasma extracellular vesicles for the diagnosis of severe acute pancreatitis.J Nanobiotechnology. 2022 Jan 28;20(1):52. doi: 10.1186/s12951-022-01239-6. J Nanobiotechnology. 2022. PMID: 35090480 Free PMC article.
-
Biomarkers to predict the severity of acute pancreatitis.Front Med (Lausanne). 2025 Aug 6;12:1619087. doi: 10.3389/fmed.2025.1619087. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40842545 Free PMC article. Review.
-
Unveiling the Potential of Extracellular Vesicles as Biomarkers and Therapeutic Nanotools for Gastrointestinal Diseases.Pharmaceutics. 2024 Apr 21;16(4):567. doi: 10.3390/pharmaceutics16040567. Pharmaceutics. 2024. PMID: 38675228 Free PMC article. Review.
-
Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis.Cell Mol Biol Lett. 2024 Apr 27;29(1):61. doi: 10.1186/s11658-024-00575-9. Cell Mol Biol Lett. 2024. PMID: 38671352 Free PMC article.
-
Fighting Fire with Fire: Exosomes and Acute Pancreatitis-Associated Acute Lung Injury.Bioengineering (Basel). 2022 Oct 26;9(11):615. doi: 10.3390/bioengineering9110615. Bioengineering (Basel). 2022. PMID: 36354526 Free PMC article. Review.
References
-
- Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. - PubMed
-
- Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig Liver Dis. 2017;49(5):540–544. - PubMed
-
- Pendharkar SA, Mathew J, Zhao J, Windsor JA, Exeter DJ, Petrov MS. Ethnic and geographic variations in the incidence of pancreatitis and post-pancreatitis diabetes mellitus in New Zealand: a nationwide population-based study. N Z Med J. 2017;130(1450):55–68. - PubMed
-
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149(6):1490–1500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

