DNA Transformations for Diagnosis and Therapy
- PMID: 33613148
- PMCID: PMC7883235
- DOI: 10.1002/adfm.202008279
DNA Transformations for Diagnosis and Therapy
Abstract
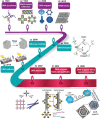
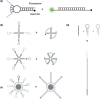
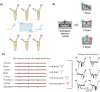
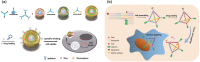
Due to its unique physical and chemical characteristics, DNA, which is known only as genetic information, has been identified and utilized as a new material at an astonishing rate. The role of DNA has increased dramatically with the advent of various DNA derivatives such as DNA-RNA, DNA-metal hybrids, and PNA, which can be organized into 2D or 3D structures by exploiting their complementary recognition. Due to its intrinsic biocompatibility, self-assembly, tunable immunogenicity, structural programmability, long stability, and electron-rich nature, DNA has generated major interest in electronic and catalytic applications. Based on its advantages, DNA and its derivatives are utilized in several fields where the traditional methodologies are ineffective. Here, the present challenges and opportunities of DNA transformations are demonstrated, especially in biomedical applications that include diagnosis and therapy. Natural DNAs previously utilized and transformed into patterns are not found in nature due to lack of multiplexing, resulting in low sensitivity and high error frequency in multi-targeted therapeutics. More recently, new platforms have advanced the diagnostic ability and therapeutic efficacy of DNA in biomedicine. There is confidence that DNA will play a strong role in next-generation clinical technology and can be used in multifaceted applications.
Keywords: DNA transformation; nanomedicine; sensing; theragnostics.
© 2020 Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
LinkOut - more resources
Full Text Sources
