Selenium at the N eural B arriers: A R eview
- PMID: 33613188
- PMCID: PMC7892976
- DOI: 10.3389/fnins.2021.630016
Selenium at the N eural B arriers: A R eview
Abstract
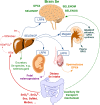
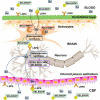
Selenium (Se) is known to contribute to several vital physiological functions in mammals: antioxidant defense, fertility, thyroid hormone metabolism, and immune response. Growing evidence indicates the crucial role of Se and Se-containing selenoproteins in the brain and brain function. As for the other essential trace elements, dietary Se needs to reach effective concentrations in the central nervous system (CNS) to exert its functions. To do so, Se-species have to cross the blood-brain barrier (BBB) and/or blood-cerebrospinal fluid barrier (BCB) of the choroid plexus. The main interface between the general circulation of the body and the CNS is the BBB. Endothelial cells of brain capillaries forming the so-called tight junctions are the primary anatomic units of the BBB, mainly responsible for barrier function. The current review focuses on Se transport to the brain, primarily including selenoprotein P/low-density lipoprotein receptor-related protein 8 (LRP8, also known as apolipoprotein E receptor-2) dependent pathway, and supplementary transport routes of Se into the brain via low molecular weight Se-species. Additionally, the potential role of Se and selenoproteins in the BBB, BCB, and neurovascular unit (NVU) is discussed. Finally, the perspectives regarding investigating the role of Se and selenoproteins in the gut-brain axis are outlined.
Keywords: LRP8; blood–brain barrier; blood–cerebrospinal fluid barrier; brain-gut axis; low molecular weight selenium species; selenium; selenium transport; selenoprotein P.
Copyright © 2021 Solovyev, Drobyshev, Blume and Michalke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

