Rapid and Reversible Development of Axonal Varicosities: A New Form of Neural Plasticity
- PMID: 33613192
- PMCID: PMC7886671
- DOI: 10.3389/fnmol.2021.610857
Rapid and Reversible Development of Axonal Varicosities: A New Form of Neural Plasticity
Abstract
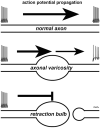
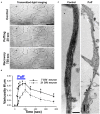
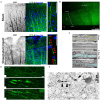
Axonal varicosities are enlarged, heterogeneous structures along axonal shafts, profoundly affecting axonal conduction and synaptic transmission. They represent a key pathological feature believed to develop via slow accumulation of axonal damage that occurs during irreversible degeneration, for example in mild traumatic brain injury (mTBI), Alzheimer's and Parkinson's diseases, and multiple sclerosis. Here this review first discusses recent in vitro results showing that axonal varicosities can be rapidly and reversibly induced by mechanical stress in cultured primary neurons from the central nervous system (CNS). This notion is further supported by in vivo studies revealing the induction of axonal varicosities across various brain regions in different mTBI mouse models, as a prominent feature of axonal pathology. Limited progress in understanding intrinsic and extrinsic regulatory mechanisms of axonal varicosity induction and development is further highlighted. Rapid and reversible formation of axonal varicosities likely plays a key role in CNS neuron mechanosensation and is a new form of neural plasticity. Future investigation in this emerging research field may reveal how to reverse axonal injury, contributing to the development of new strategies for treating brain injuries and related neurodegenerative diseases.
Keywords: Alzheimer's disease; action potential; axonal varicosity; mechanosensitive ion channel; microtubule; mild traumatic brain injury; neural plasticity; synaptic transmission.
Copyright © 2021 Gu.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation.Acta Neuropathol Commun. 2022 Jan 24;10(1):7. doi: 10.1186/s40478-022-01309-8. Acta Neuropathol Commun. 2022. PMID: 35074017 Free PMC article.
-
A Microbiomechanical System for Studying Varicosity Formation and Recovery in Central Neuron Axons.J Vis Exp. 2018 Apr 30;(134):57202. doi: 10.3791/57202. J Vis Exp. 2018. PMID: 29757278 Free PMC article.
-
Polarity of varicosity initiation in central neuron mechanosensation.J Cell Biol. 2017 Jul 3;216(7):2179-2199. doi: 10.1083/jcb.201606065. Epub 2017 Jun 12. J Cell Biol. 2017. PMID: 28606925 Free PMC article.
-
Mechanisms of Pathological Axonal Degeneration.In: Tran TS, Yaron A, editors. Wiring the Nervous System: Mechanisms of Axonal and Dendritic Remodelling in Health and Disease. 1st edition. Abingdon: River Publishers; 2024 Jan 31. Chapter 6. In: Tran TS, Yaron A, editors. Wiring the Nervous System: Mechanisms of Axonal and Dendritic Remodelling in Health and Disease. 1st edition. Abingdon: River Publishers; 2024 Jan 31. Chapter 6. PMID: 40036397 Free Books & Documents. Review.
-
Axonal varicosity distributions along parallel fibers: a new angle on a cerebellar circuit.Cerebellum. 2003;2(2):110-3. doi: 10.1080/14734220310011407. Cerebellum. 2003. PMID: 12880178 Review.
Cited by
-
Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation.Acta Neuropathol Commun. 2022 Jan 24;10(1):7. doi: 10.1186/s40478-022-01309-8. Acta Neuropathol Commun. 2022. PMID: 35074017 Free PMC article.
-
A Brief Review of In Vitro Models for Injury and Regeneration in the Peripheral Nervous System.Int J Mol Sci. 2022 Jan 13;23(2):816. doi: 10.3390/ijms23020816. Int J Mol Sci. 2022. PMID: 35055003 Free PMC article. Review.
-
Cocaine-regulated trafficking of dopamine transporters in cultured neurons revealed by a pH sensitive reporter.iScience. 2022 Dec 9;26(1):105782. doi: 10.1016/j.isci.2022.105782. eCollection 2023 Jan 20. iScience. 2022. PMID: 36594015 Free PMC article.
-
Raloxifene Modulates Microglia and Rescues Visual Deficits and Pathology After Impact Traumatic Brain Injury.Front Neurosci. 2021 Oct 29;15:701317. doi: 10.3389/fnins.2021.701317. eCollection 2021. Front Neurosci. 2021. PMID: 34776838 Free PMC article.
-
SARS-CoV-2 S1 Protein Induces Endolysosome Dysfunction and Neuritic Dystrophy.Front Cell Neurosci. 2021 Oct 27;15:777738. doi: 10.3389/fncel.2021.777738. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34776872 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

