A Long-Term Enriched Environment Ameliorates the Accelerated Age-Related Memory Impairment Induced by Gestational Administration of Lipopolysaccharide: Role of Plastic Mitochondrial Quality Control
- PMID: 33613195
- PMCID: PMC7886998
- DOI: 10.3389/fncel.2020.559182
A Long-Term Enriched Environment Ameliorates the Accelerated Age-Related Memory Impairment Induced by Gestational Administration of Lipopolysaccharide: Role of Plastic Mitochondrial Quality Control
Abstract
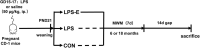
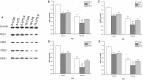
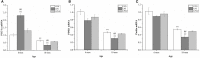
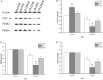
Studies have shown that gestational inflammation accelerates age-related memory impairment in mother mice. An enriched environment (EE) can improve age-related memory impairment, whereas mitochondrial dysfunction has been implicated in the pathogenesis of brain aging. However, it is unclear whether an EE can counteract the accelerated age-related memory impairment induced by gestational inflammation and whether this process is associated with the disruption of mitochondrial quality control (MQC) processes. In this study, CD-1 mice received daily intraperitoneal injections of lipopolysaccharide (LPS, 50 μg/kg) or normal saline (CON group) during gestational days 15-17 and were separated from their offspring at the end of normal lactation. The mothers that received LPS were divided into LPS group and LPS plus EE (LPS-E) treatment groups based on whether the mice were exposed to an EE until the end of the experiment. At 6 and 18 months of age, the Morris water maze test was used to evaluate spatial learning and memory abilities. Quantitative reverse transcription polymerase chain reaction and Western blot were used to measure the messenber RNA (mRNA) and protein levels of MQC-related genes in the hippocampus, respectively. The results showed that all the aged (18 months old) mice underwent a striking decline in spatial learning and memory performances and decreased mRNA/protein levels related to mitochondrial dynamics (Mfn1/Mfn2, OPA1, and Drp1), biogenesis (PGC-1α), and mitophagy (PINK1/parkin) in the hippocampi compared with the young (6 months old) mice. LPS treatment exacerbated the decline in age-related spatial learning and memory and enhanced the reduction in the mRNA and protein levels of MQC-related genes but increased the levels of PGC-1α in young mice. Exposure to an EE could alleviate the accelerated decline in age-related spatial learning and memory abilities and the accelerated changes in MQC-related mRNA or protein levels resulting from LPS treatment, especially in aged mice. In conclusion, long-term exposure to an EE can counteract the accelerated age-related spatial cognition impairment modulated by MQC in CD-1 mother mice that experience inflammation during pregnancy.
Keywords: age-related memory impairment; enriched environment; hippocampus; lipopolysaccharide; mitochondrial quality control.
Copyright © 2021 Zhuang, Zhang, Zhang, Ge, Sun, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A postpartum enriched environment rescues impaired cognition and oxidative markers in aged mice with gestational inflammation.Brain Behav. 2022 Dec;12(12):e2817. doi: 10.1002/brb3.2817. Epub 2022 Nov 21. Brain Behav. 2022. PMID: 36409568 Free PMC article.
-
Long-Term Environmental Enrichment Relieves Dysfunctional Cognition and Synaptic Protein Levels Induced by Prenatal Inflammation in Older CD-1 Mice.Neural Plast. 2022 May 6;2022:1483101. doi: 10.1155/2022/1483101. eCollection 2022. Neural Plast. 2022. PMID: 35574247 Free PMC article.
-
Environmental enrichment improves declined cognition induced by prenatal inflammatory exposure in aged CD-1 mice: Role of NGPF2 and PSD-95.Front Aging Neurosci. 2022 Nov 21;14:1021237. doi: 10.3389/fnagi.2022.1021237. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36479357 Free PMC article.
-
Effects of gestational inflammation on age-related cognitive decline and hippocampal Gdnf-GFRα1 levels in F1 and F2 generations of CD-1 Mice.BMC Neurosci. 2023 Apr 13;24(1):26. doi: 10.1186/s12868-023-00793-5. BMC Neurosci. 2023. PMID: 37055728 Free PMC article.
-
Novel insights into exhaustive exercise-induced myocardial injury: Focusing on mitochondrial quality control.Front Cardiovasc Med. 2022 Oct 14;9:1015639. doi: 10.3389/fcvm.2022.1015639. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312267 Free PMC article. Review.
Cited by
-
Mitochondrial antioxidant elamipretide improves learning and memory impairment induced by chronic sleep deprivation in mice.Brain Behav. 2024 May;14(5):e3508. doi: 10.1002/brb3.3508. Brain Behav. 2024. PMID: 38688894 Free PMC article.
-
A postpartum enriched environment rescues impaired cognition and oxidative markers in aged mice with gestational inflammation.Brain Behav. 2022 Dec;12(12):e2817. doi: 10.1002/brb3.2817. Epub 2022 Nov 21. Brain Behav. 2022. PMID: 36409568 Free PMC article.
-
An enriched environment ameliorates maternal sleep deprivation-induced cognitive impairment in aged mice by improving mitochondrial function via the Sirt1/PGC-1α pathway.Aging (Albany NY). 2024 Jan 16;16(2):1128-1144. doi: 10.18632/aging.205385. Epub 2024 Jan 16. Aging (Albany NY). 2024. PMID: 38231482 Free PMC article.
-
Subsequent maternal sleep deprivation aggravates neurobehavioral abnormalities, inflammation, and synaptic function in adult male mice exposed to prenatal inflammation.Front Behav Neurosci. 2023 Jul 25;17:1226300. doi: 10.3389/fnbeh.2023.1226300. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37560531 Free PMC article.
-
Long-Term Environmental Enrichment Relieves Dysfunctional Cognition and Synaptic Protein Levels Induced by Prenatal Inflammation in Older CD-1 Mice.Neural Plast. 2022 May 6;2022:1483101. doi: 10.1155/2022/1483101. eCollection 2022. Neural Plast. 2022. PMID: 35574247 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

