Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions
- PMID: 33613196
- PMCID: PMC7892457
- DOI: 10.3389/fncel.2020.612480
Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions
Abstract
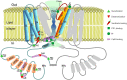
Transient receptor potential vanilloid 1 (TRPV1) is a calcium-permeable ion channel best known for its ability to be gated by the pungent constituent of red chili pepper, capsaicin, and related chemicals from the group of vanilloids as well as by noxious heat. As such, it is mostly expressed in sensory neurons to act as a detector of painful stimuli produced by pungent chemicals and high temperatures. Its activation is also sensitized by the numerous endogenous inflammatory mediators and second messengers, making it an important determinant of nociceptive signaling. Except for such signaling, though, neuronal TRPV1 activation may influence various organ functions by promoting the release of bioactive neuropeptides from sensory fiber innervation organs. However, TRPV1 is also found outside the sensory nervous system in which its activation and function is not that straightforward. Thus, TRPV1 expression is detected in skeletal muscle; in some types of smooth muscle; in epithelial and immune cells; and in adipocytes, where it can be activated by the combination of dietary vanilloids, endovanilloids, and pro-inflammatory factors while the intracellular calcium signaling that this initiates can regulate processes as diverse as muscle constriction, cell differentiation, and carcinogenesis. The purpose of the present review is to provide a clear-cut distinction between neurogenic TRPV1 effects in various tissues consequent to its activation in sensory nerve endings and non-neurogenic TRPV1 effects due to its expression in cell types other than sensory neurons.
Keywords: TRPV1; adipocytes; epithelia cells; sensory neuron; smooth muscle.
Copyright © 2021 Shuba.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Contribution of transient receptor potential vanilloid 1 (TRPV1) channel to cholinergic contraction of rat bladder smooth muscle.J Physiol. 2024 Aug;602(15):3693-3713. doi: 10.1113/JP285514. Epub 2024 Jul 6. J Physiol. 2024. PMID: 38970617
-
TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia.Eur J Pharmacol. 2004 Oct 1;500(1-3):231-41. doi: 10.1016/j.ejphar.2004.07.028. Eur J Pharmacol. 2004. PMID: 15464036 Review.
-
Endogenous and Exogenous Vanilloids Evoke Disparate TRPV1 Activation to Produce Distinct Neuronal Responses.Front Pharmacol. 2020 Jun 12;11:903. doi: 10.3389/fphar.2020.00903. eCollection 2020. Front Pharmacol. 2020. PMID: 32595512 Free PMC article.
-
Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation.Biochemistry. 2006 Apr 18;45(15):4915-26. doi: 10.1021/bi052082f. Biochemistry. 2006. PMID: 16605259 Free PMC article.
-
Capsaicin receptor: TRPV1 a promiscuous TRP channel.Handb Exp Pharmacol. 2007;(179):155-71. doi: 10.1007/978-3-540-34891-7_9. Handb Exp Pharmacol. 2007. PMID: 17217056 Review.
Cited by
-
Capsaicin: Emerging Pharmacological and Therapeutic Insights.Curr Issues Mol Biol. 2024 Jul 24;46(8):7895-7943. doi: 10.3390/cimb46080468. Curr Issues Mol Biol. 2024. PMID: 39194685 Free PMC article. Review.
-
Targeting CB2 and TRPV1: Computational Approaches for the Identification of Dual Modulators.Front Mol Biosci. 2022 Feb 25;9:841190. doi: 10.3389/fmolb.2022.841190. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281260 Free PMC article.
-
Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and trkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion.Int J Mol Sci. 2022 Mar 26;23(7):3633. doi: 10.3390/ijms23073633. Int J Mol Sci. 2022. PMID: 35408995 Free PMC article.
-
The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension?Int J Mol Sci. 2023 May 15;24(10):8769. doi: 10.3390/ijms24108769. Int J Mol Sci. 2023. PMID: 37240118 Free PMC article. Review.
-
TRPV1 and mast cell involvement in repeated variate stress-induced urinary bladder dysfunction in adult female mice.Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F476-F488. doi: 10.1152/ajprenal.00125.2024. Epub 2024 Jul 11. Am J Physiol Renal Physiol. 2024. PMID: 38991005 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

