PKN1 Is a Novel Regulator of Hippocampal GluA1 Levels
- PMID: 33613259
- PMCID: PMC7892898
- DOI: 10.3389/fnsyn.2021.640495
PKN1 Is a Novel Regulator of Hippocampal GluA1 Levels
Abstract
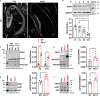
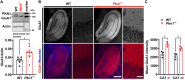
Alterations in the processes that control α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) expression, assembly and trafficking are closely linked to psychiatric and neurodegenerative disorders. We have recently shown that the serine/threonine kinase Protein kinase N1 (PKN1) is a developmentally active regulator of cerebellar synaptic maturation by inhibiting AKT and the neurogenic transcription factor neurogenic differentiation factor-2 (NeuroD2). NeuroD2 is involved in glutamatergic synaptic maturation by regulating expression levels of various synaptic proteins. Here we aimed to study the effect of Pkn1 knockout on AKT phosphorylation and NeuroD2 levels in the hippocampus and the subsequent expression levels of the NeuroD2 targets and AMPAR subunits: glutamate receptor 1 (GluA1) and GluA2/3. We show that PKN1 is expressed throughout the hippocampus. Interestingly, not only postnatal but also adult hippocampal phospho-AKT and NeuroD2 levels were significantly elevated upon Pkn1 knockout. Postnatal and adult Pkn1 -/- hippocampi showed enhanced expression of the AMPAR subunit GluA1, particularly in area CA1. Surprisingly, GluA2/3 levels were not different between both genotypes. In addition to higher protein levels, we also found an enhanced GluA1 content in the membrane fraction of postnatal and adult Pkn1 -/- animals, while GluA2/3 levels remained unchanged. This points toward a very specific regulation of GluA1 expression and/or trafficking by the novel PKN1-AKT-NeuroD2 axis. Considering the important role of GluA1 in hippocampal development as well as the pathophysiology of several disorders, ranging from Alzheimer's, to depression and schizophrenia, our results validate PKN1 for future studies into neurological disorders related to altered AMPAR subunit expression in the hippocampus.
Keywords: AMPA receptor; GluA1; NeuroD2; PKN1; hippocampus.
Copyright © 2021 Safari, Obexer, Baier-Bitterlich and zur Nedden.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Novel Regulation of the Synthesis of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Subunit GluA1 by Carnitine Palmitoyltransferase 1C (CPT1C) in the Hippocampus.J Biol Chem. 2015 Oct 16;290(42):25548-60. doi: 10.1074/jbc.M115.681064. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338711 Free PMC article.
-
Role of PKN1 in Retinal Cell Type Formation.Int J Mol Sci. 2024 Feb 29;25(5):2848. doi: 10.3390/ijms25052848. Int J Mol Sci. 2024. PMID: 38474095 Free PMC article.
-
Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica.Biomed Pharmacother. 2019 Feb;110:168-180. doi: 10.1016/j.biopha.2018.11.044. Epub 2018 Nov 20. Biomed Pharmacother. 2019. PMID: 30469081
-
Protein kinase N1 critically regulates cerebellar development and long-term function.J Clin Invest. 2018 May 1;128(5):2076-2088. doi: 10.1172/JCI96165. Epub 2018 Apr 16. J Clin Invest. 2018. PMID: 29494346 Free PMC article.
-
Dynamic increases in AMPA receptor phosphorylation in the rat hippocampus in response to amphetamine.J Neurochem. 2015 Jun;133(6):795-805. doi: 10.1111/jnc.13067. Epub 2015 Mar 2. J Neurochem. 2015. PMID: 25689263 Free PMC article.
Cited by
-
PKN1 Exerts Neurodegenerative Effects in an In Vitro Model of Cerebellar Hypoxic-Ischemic Encephalopathy via Inhibition of AKT/GSK3β Signaling.Biomolecules. 2023 Oct 31;13(11):1599. doi: 10.3390/biom13111599. Biomolecules. 2023. PMID: 38002281 Free PMC article.
-
Glucose-1,6-bisphosphate: A new gatekeeper of cerebral mitochondrial pyruvate uptake.Mol Metab. 2024 Oct;88:102018. doi: 10.1016/j.molmet.2024.102018. Epub 2024 Aug 24. Mol Metab. 2024. PMID: 39182844 Free PMC article.
-
Developmental pyrethroid exposure disrupts molecular pathways for MAP kinase and circadian rhythms in mouse brain.Physiol Genomics. 2025 Apr 1;57(4):240-253. doi: 10.1152/physiolgenomics.00033.2024. Epub 2025 Feb 17. Physiol Genomics. 2025. PMID: 39961078 Free PMC article.
-
Mapping the hippocampal spatial proteomic signature in male and female mice of an early Alzheimer's disease model.Biol Sex Differ. 2025 May 25;16(1):36. doi: 10.1186/s13293-025-00697-5. Biol Sex Differ. 2025. PMID: 40414897 Free PMC article.
-
Developmental pyrethroid exposure disrupts molecular pathways for MAP kinase and circadian rhythms in mouse brain.bioRxiv [Preprint]. 2024 Mar 11:2023.08.28.555113. doi: 10.1101/2023.08.28.555113. bioRxiv. 2024. Update in: Physiol Genomics. 2025 Apr 01;57(4):240-253. doi: 10.1152/physiolgenomics.00033.2024. PMID: 37745438 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

