Ectonucleotidases in Acute and Chronic Inflammation
- PMID: 33613285
- PMCID: PMC7887318
- DOI: 10.3389/fphar.2020.619458
Ectonucleotidases in Acute and Chronic Inflammation
Abstract
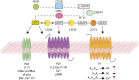
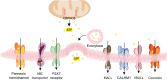
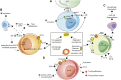
Ectonucleotidases are extracellular enzymes with a pivotal role in inflammation that hydrolyse extracellular purine and pyrimidine nucleotides, e.g., ATP, UTP, ADP, UDP, AMP and NAD+. Ectonucleotidases, expressed by virtually all cell types, immune cells included, either as plasma membrane-associated or secreted enzymes, are classified into four main families: 1) nucleoside triphosphate diphosphohydrolases (NTPDases), 2) nicotinamide adenine dinucleotide glycohydrolase (NAD glycohydrolase/ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1), 3) ecto-5'-nucleotidase (NT5E), and 4) ecto-nucleotide pyrophosphatase/phosphodiesterases (NPPs). Concentration of ATP, UTP and NAD+ can be increased in the extracellular space thanks to un-regulated, e.g., cell damage or cell death, or regulated processes. Regulated processes include secretory exocytosis, connexin or pannexin hemichannels, ATP binding cassette (ABC) transporters, calcium homeostasis modulator (CALMH) channels, the ATP-gated P2X7 receptor, maxi-anion channels (MACs) and volume regulated ion channels (VRACs). Hydrolysis of extracellular purine nucleotides generates adenosine, an important immunosuppressant. Extracellular nucleotides and nucleosides initiate or dampen inflammation via P2 and P1 receptors, respectively. All these agents, depending on their level of expression or activation and on the agonist concentration, are potent modulators of inflammation and key promoters of host defences, immune cells activation, pathogen clearance, tissue repair and regeneration. Thus, their knowledge is of great importance for a full understanding of the pathophysiology of acute and chronic inflammatory diseases. A selection of these pathologies will be briefly discussed here.
Keywords: ATP; acute inflammation; chronic inflammatory diseases; ecto-nucleotidases; immune cells; purinergic receptors; tumors.
Copyright © 2021 Giuliani, Sarti and Di Virgilio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Ectonucleotidases and nucleotide/nucleoside transporters as pharmacological targets for neurological disorders.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):739-50. doi: 10.2174/187152712803581092. CNS Neurol Disord Drug Targets. 2012. PMID: 22963442 Review.
-
Mechanisms regulating airway nucleotides.Subcell Biochem. 2011;55:17-49. doi: 10.1007/978-94-007-1217-1_2. Subcell Biochem. 2011. PMID: 21560043 Review.
-
Extracellular nucleotides and nucleosides as signalling molecules.Immunol Lett. 2019 Jan;205:16-24. doi: 10.1016/j.imlet.2018.11.006. Epub 2018 Nov 12. Immunol Lett. 2019. PMID: 30439478 Review.
-
Impact of ectonucleotidases in autonomic nervous functions.Auton Neurosci. 2015 Sep;191:25-38. doi: 10.1016/j.autneu.2015.04.014. Epub 2015 May 11. Auton Neurosci. 2015. PMID: 26008223 Review.
-
Extracellular ATP: A Feasible Target for Cancer Therapy.Cells. 2020 Nov 17;9(11):2496. doi: 10.3390/cells9112496. Cells. 2020. PMID: 33212982 Free PMC article. Review.
Cited by
-
In Search of a Role for Extracellular Purine Enzymes in Bone Function.Biomolecules. 2021 Apr 30;11(5):679. doi: 10.3390/biom11050679. Biomolecules. 2021. PMID: 33946568 Free PMC article. Review.
-
The immunomodulatory ballet of tumour-derived extracellular vesicles and neutrophils orchestrating the dynamic CD73/PD-L1 pathway in cancer.J Extracell Vesicles. 2024 Jul;13(7):e12480. doi: 10.1002/jev2.12480. J Extracell Vesicles. 2024. PMID: 38978304 Free PMC article.
-
Crosstalk between P2Y receptors and cyclooxygenase activity in inflammation and tissue repair.Purinergic Signal. 2024 Apr;20(2):145-155. doi: 10.1007/s11302-023-09938-x. Epub 2023 Apr 13. Purinergic Signal. 2024. PMID: 37052777 Free PMC article. Review.
-
The Phagocytic Code Regulating Phagocytosis of Mammalian Cells.Front Immunol. 2021 Jun 9;12:629979. doi: 10.3389/fimmu.2021.629979. eCollection 2021. Front Immunol. 2021. PMID: 34177884 Free PMC article. Review.
-
Hepatic extracellular ATP/adenosine dynamics in zebrafish models of alcoholic and metabolic steatotic liver disease.Sci Rep. 2024 Apr 3;14(1):7813. doi: 10.1038/s41598-024-58043-5. Sci Rep. 2024. PMID: 38565862 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

