Conditioned Media of Choroid Plexus Epithelium Cells Attenuates High Pi-Induced Calcification of MOVAS Cells by Inhibiting ROS-Mediated Signal Pathways
- PMID: 33613308
- PMCID: PMC7892975
- DOI: 10.3389/fphys.2021.607739
Conditioned Media of Choroid Plexus Epithelium Cells Attenuates High Pi-Induced Calcification of MOVAS Cells by Inhibiting ROS-Mediated Signal Pathways
Abstract
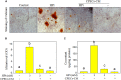
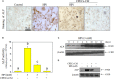
Vascular calcification was an independent risk of cardiovascular and cerebrovascular diseases (CCDs). Studies reported that conditioned media of choroid plexus epithelium cells (CPECs-CM) showed potential neuroprotective effects. However, the protective effect of CPECs-CM against vascular calcification (VC) has not been reported yet. Herein, high phosphate (HPi)-induced calcification model in mouse aortic vascular smooth muscle cells (MOVAS) was established, and the protective effects and underlying mechanism of CPECs-CM against HPi-induced calcification were explored. The results indicated that CPEC cells were successfully isolated and cultured, and CPECs-CM co-treatment significantly inhibited HPi-induced calcification of MOVAS cells through blocking alkaline phosphatase activity and expression. CPECs-CM co-treatment also suppressed reactive oxide species-mediated DNA damage in HPi-treated MOVAS cells. Moreover, dysfunction of MAPKs and PI3K/AKT pathways both contributed to HPi-induced calcification of MOVAS cells, and CPECs-CM co-treatment attenuated HPi-induced calcification by normalizing MAPKs and PI3K/AKT expression. Taken together, our findings provide evidence that CPECs-CM had the potential to inhibit vascular calcification with potent application in chemoprevention and chemotherapy of human CCD.
Keywords: DNA damage; MAPKs and PI3K/AKT; ROS; choroid plexus epithelium cells; vascular calcification.
Copyright © 2021 Hui, Wang, Zhang, Liu, Wang, Hu, Zhang, Zhao, Geng, Wang and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Apelin-13 attenuates high glucose-induced calcification of MOVAS cells by regulating MAPKs and PI3K/AKT pathways and ROS-mediated signals.Biomed Pharmacother. 2020 Aug;128:110271. doi: 10.1016/j.biopha.2020.110271. Epub 2020 May 22. Biomed Pharmacother. 2020. PMID: 32450527
-
Selenium-Containing Protein From Selenium-Enriched Spirulina platensis Attenuates High Glucose-Induced Calcification of MOVAS Cells by Inhibiting ROS-Mediated DNA Damage and Regulating MAPK and PI3K/AKT Pathways.Front Physiol. 2020 Jul 9;11:791. doi: 10.3389/fphys.2020.00791. eCollection 2020. Front Physiol. 2020. PMID: 32733280 Free PMC article.
-
Conditioned media of choroid plexus epithelial cells induces Nrf2-activated phase II antioxidant response proteins and suppresses oxidative stress-induced apoptosis in PC12 cells.J Mol Neurosci. 2014 Aug;53(4):617-25. doi: 10.1007/s12031-014-0228-4. Epub 2014 Feb 4. J Mol Neurosci. 2014. PMID: 24488602
-
Comparison of the Inhibitory Mechanisms of Diethyl Citrate, Sodium Citrate, and Phosphonoformic Acid on Calcification Induced by High Inorganic Phosphate Contents in Mouse Aortic Smooth Muscle Cells.J Cardiovasc Pharmacol. 2017 Dec;70(6):411-419. doi: 10.1097/FJC.0000000000000537. J Cardiovasc Pharmacol. 2017. PMID: 28902664
-
Vascular Calcification Mechanisms: Updates and Renewed Insight into Signaling Pathways Involved in High Phosphate-Mediated Vascular Smooth Muscle Cell Calcification.Biomedicines. 2021 Jul 12;9(7):804. doi: 10.3390/biomedicines9070804. Biomedicines. 2021. PMID: 34356868 Free PMC article. Review.
References
-
- Aliaghaei A., Khodagholi F., Ahmadiani A. (2014). Conditioned media of choroid plexus epithelial cells induces Nrf2-activated phase II antioxidant response proteins and suppresses oxidative stress-induced apoptosis in PC12 cells. J. Mol. Neurosci. 53 617–625. 10.1007/s12031-014-0228-4 - DOI - PubMed
-
- Borlongan C. V., Skinner S. J., Geaney M., Vasconcellos A. V., Elliott R. B., Emerich D. F. (2004). CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport 15 1543–1547. 10.1097/01.wnr.0000133298.84901.cf - DOI - PubMed
-
- Choi S. Y., Ryu H. M., Oh E. J., Choi J. Y., Cho J. H., Kim C. D., et al. (2017). Dipeptidyl peptidase-4 inhibitor gemigliptin protects against vascular calcification in an experimental chronic kidney disease and vascular smooth muscle cells. PLoS One 12:e0180393. 10.1371/journal.pone.0180393 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

