Gastrointestinal Helminth Infection Improves Insulin Sensitivity, Decreases Systemic Inflammation, and Alters the Composition of Gut Microbiota in Distinct Mouse Models of Type 2 Diabetes
- PMID: 33613446
- PMCID: PMC7892786
- DOI: 10.3389/fendo.2020.606530
Gastrointestinal Helminth Infection Improves Insulin Sensitivity, Decreases Systemic Inflammation, and Alters the Composition of Gut Microbiota in Distinct Mouse Models of Type 2 Diabetes
Abstract
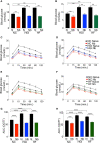
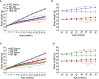
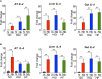
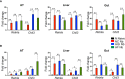
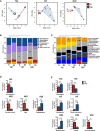
Type 2 diabetes (T2D) is a major health problem and is considered one of the top 10 diseases leading to death globally. T2D has been widely associated with systemic and local inflammatory responses and with alterations in the gut microbiota. Microorganisms, including parasitic worms and gut microbes have exquisitely co-evolved with their hosts to establish an immunological interaction that is essential for the formation and maintenance of a balanced immune system, including suppression of excessive inflammation. Herein we show that both prophylactic and therapeutic infection of mice with the parasitic hookworm-like nematode, Nippostrongylus brasiliensis, significantly reduced fasting blood glucose, oral glucose tolerance and body weight gain in two different diet-induced mouse models of T2D. Helminth infection was associated with elevated type 2 immune responses including increased eosinophil numbers in the mesenteric lymph nodes, liver and adipose tissues, as well as increased expression of IL-4 and alternatively activated macrophage marker genes in adipose tissue, liver and gut. N. brasiliensis infection was also associated with significant compositional changes in the gut microbiota at both the phylum and order levels. Our findings show that N. brasiliensis infection drives changes in local and systemic immune cell populations, and that these changes are associated with a reduction in systemic and local inflammation and compositional changes in the gut microbiota which cumulatively might be responsible for the improved insulin sensitivity observed in infected mice. Our findings indicate that carefully controlled therapeutic hookworm infection in humans could be a novel approach for treating metabolic syndrome and thereby preventing T2D.
Keywords: M2 macrophages; Nippostrongylus brasiliensis; eosinophils; helminth; high fat diet; high glycemic index diet; microbiota; type 2 diabetes.
Copyright © 2021 Khudhair, Alhallaf, Eichenberger, Whan, Kupz, Field, Krause, Wilson, Daly, Giacomin, Sotillo and Loukas.
Conflict of interest statement
Author LK was employed by company Microba Pty Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
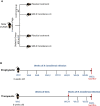

Similar articles
-
Administration of Hookworm Excretory/Secretory Proteins Improves Glucose Tolerance in a Mouse Model of Type 2 Diabetes.Biomolecules. 2022 Apr 26;12(5):637. doi: 10.3390/biom12050637. Biomolecules. 2022. PMID: 35625566 Free PMC article.
-
Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice.FASEB J. 2015 Jul;29(7):3027-39. doi: 10.1096/fj.14-266239. Epub 2015 Apr 7. FASEB J. 2015. PMID: 25852044
-
Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota.Diabetologia. 2018 Jun;61(6):1447-1458. doi: 10.1007/s00125-018-4583-5. Epub 2018 Mar 3. Diabetologia. 2018. PMID: 29502266 Free PMC article.
-
Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes.Diabetes Metab Res Rev. 2015 Sep;31(6):545-61. doi: 10.1002/dmrr.2617. Epub 2014 Dec 8. Diabetes Metab Res Rev. 2015. PMID: 25352002 Review.
-
Role of the gut microbiota in type 2 diabetes and related diseases.Metabolism. 2021 Apr;117:154712. doi: 10.1016/j.metabol.2021.154712. Epub 2021 Jan 23. Metabolism. 2021. PMID: 33497712 Review.
Cited by
-
Microbiome analysis reveals the effects of black soldier fly oil on gut microbiota in pigeon.Front Microbiol. 2022 Sep 6;13:998524. doi: 10.3389/fmicb.2022.998524. eCollection 2022. Front Microbiol. 2022. PMID: 36160221 Free PMC article.
-
Infections and immunity: associations with obesity and related metabolic disorders.J Pathol Transl Med. 2023 Jan;57(1):28-42. doi: 10.4132/jptm.2022.11.14. Epub 2023 Jan 15. J Pathol Transl Med. 2023. PMID: 36647284 Free PMC article. Review.
-
Helminths in alternative therapeutics of inflammatory bowel disease.Intest Res. 2025 Jan;23(1):8-22. doi: 10.5217/ir.2023.00059. Epub 2024 Jan 12. Intest Res. 2025. PMID: 39916482 Free PMC article. Review.
-
Association of Strongyloides stercoralis infection and type 2 diabetes mellitus in northeastern Thailand: Impact on diabetic complication-related renal biochemical parameters.PLoS One. 2022 May 31;17(5):e0269080. doi: 10.1371/journal.pone.0269080. eCollection 2022. PLoS One. 2022. PMID: 35639713 Free PMC article.
-
Food for thought - ILC metabolism in the context of helminth infections.Mucosal Immunol. 2022 Jun;15(6):1234-1242. doi: 10.1038/s41385-022-00559-y. Epub 2022 Aug 31. Mucosal Immunol. 2022. PMID: 36045216 Free PMC article. Review.
References
-
- Federation ID IDF diabetes atlas. Eighth Brussels, Belgium: International Diabetes Federation; (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

