Signal Transduction Mechanisms for Glucagon-Induced Somatolactin Secretion and Gene Expression in Nile Tilapia (Oreochromis niloticus) Pituitary Cells
- PMID: 33613457
- PMCID: PMC7890253
- DOI: 10.3389/fendo.2020.629077
Signal Transduction Mechanisms for Glucagon-Induced Somatolactin Secretion and Gene Expression in Nile Tilapia (Oreochromis niloticus) Pituitary Cells
Abstract
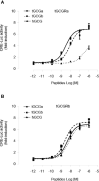
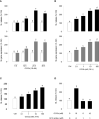
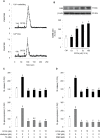
Glucagon (GCG) plays a stimulatory role in pituitary hormone regulation, although previous studies have not defined the molecular mechanism whereby GCG affects pituitary hormone secretion. To this end, we identified two distinct proglucagons, Gcga and Gcgb, as well as GCG receptors, Gcgra and Gcgrb, in Nile tilapia (Oreochromis niloticus). Using the cAMP response element (CRE)-luciferase reporter system, tilapia GCGa and GCGb could reciprocally activate the two GCG receptors expressed in human embryonic kidney 293 (HEK293) cells. Quantitative real-time PCR analysis revealed that differential expression of the Gcga and Gcgb and their cognate receptors Gcgra and Gcgrb was found in the various tissues of tilapia. In particular, the Gcgrb is abundantly expressed in the neurointermediate lobe (NIL) of the pituitary gland. In primary cultures of tilapia NIL cells, GCGb effectively stimulated SL release, with parallel rises in the mRNA levels, and co-incubation with the GCG antagonist prevented GCGb-stimulated SL release. In parallel experiments, GCGb treatment dose-dependently enhanced intracellular cyclic adenosine monophosphate (cAMP) accumulation with increasing inositol 1,4,5-trisphosphate (IP3) concentration and the resulting in transient increases of Ca2+ signals in the primary NIL cell culture. Using selective pharmacological approaches, the adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) and phospholipase C (PLC)/IP3/Ca2+/calmodulin (CaM)/CaMK-II pathways were shown to be involved in GCGb-induced SL release and mRNA expression. Together, these results provide evidence for the first time that GCGb can act at the pituitary level to stimulate SL release and gene expression via GCGRb through the activation of the AC/cAMP/PKA and PLC/IP3/Ca2+/CaM/CaMK-II cascades.
Keywords: glucagon; pituitary; secretion and gene expression; somatolactin; tilapia.
Copyright © 2021 Zhang, Lian, Xu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Grass carp somatolactin: II. Pharmacological study on postreceptor signaling mechanisms for PACAP-induced somatolactin-alpha and -beta gene expression.Am J Physiol Endocrinol Metab. 2008 Aug;295(2):E477-90. doi: 10.1152/ajpendo.90386.2008. Epub 2008 Jun 3. Am J Physiol Endocrinol Metab. 2008. PMID: 18523121
-
Dopamine inhibits somatolactin gene expression in tilapia pituitary cells through the dopamine D2 receptors.Comp Biochem Physiol A Mol Integr Physiol. 2016 Jul;197:35-42. doi: 10.1016/j.cbpa.2016.03.008. Epub 2016 Mar 10. Comp Biochem Physiol A Mol Integr Physiol. 2016. PMID: 26970582
-
Somatostatin-28 inhibitory action on somatolactin-α and -β gene expression in goldfish.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R755-68. doi: 10.1152/ajpregu.00193.2014. Epub 2014 Jul 9. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25009216
-
Regulation of gonadotropin subunit genes in tilapia.Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):489-502. doi: 10.1016/s1096-4959(01)00345-1. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11399484 Review.
-
Pituitary adenylate cyclase activating polypeptide as a novel hypophysiotropic factor in fish.Biochem Cell Biol. 2000;78(3):329-43. Biochem Cell Biol. 2000. PMID: 10949084 Review.
Cited by
-
Calcium/Calmodulin-Dependent Kinases in the Hypothalamus, Pituitary, and Pineal Gland: An Overview.Int J Endocrinol. 2022 Dec 26;2022:1103346. doi: 10.1155/2022/1103346. eCollection 2022. Int J Endocrinol. 2022. PMID: 36601542 Free PMC article. Review.
-
Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge.Vaccines (Basel). 2021 Oct 29;9(11):1253. doi: 10.3390/vaccines9111253. Vaccines (Basel). 2021. PMID: 34835184 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

