Plant Glycan Metabolism by Bifidobacteria
- PMID: 33613480
- PMCID: PMC7889515
- DOI: 10.3389/fmicb.2021.609418
Plant Glycan Metabolism by Bifidobacteria
Abstract
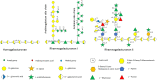
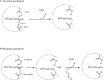
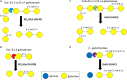
Members of the genus Bifidobacterium, of which the majority have been isolated as gut commensals, are Gram-positive, non-motile, saccharolytic, non-sporulating, anaerobic bacteria. Many bifidobacterial strains are considered probiotic and therefore are thought to bestow health benefits upon their host. Bifidobacteria are highly abundant among the gut microbiota of healthy, full term, breast-fed infants, yet the relative average abundance of bifidobacteria tends to decrease as the human host ages. Because of the inverse correlation between bifidobacterial abundance/prevalence and health, there has been an increasing interest in maintaining, increasing or restoring bifidobacterial populations in the infant, adult and elderly gut. In order to colonize and persist in the gastrointestinal environment, bifidobacteria must be able to metabolise complex dietary and/or host-derived carbohydrates, and be resistant to various environmental challenges of the gut. This is not only important for the autochthonous bifidobacterial species colonising the gut, but also for allochthonous bifidobacteria provided as probiotic supplements in functional foods. For example, Bifidobacterium longum subsp. longum is a taxon associated with the metabolism of plant-derived poly/oligosaccharides in the adult diet, being capable of metabolising hemicellulose and various pectin-associated glycans. Many of these plant glycans are believed to stimulate the metabolism and growth of specific bifidobacterial species and are for this reason classified as prebiotics. In this review, bifidobacterial carbohydrate metabolism, with a focus on plant poly-/oligosaccharide degradation and uptake, as well as its associated regulation, will be discussed.
Keywords: CAZy enzymes; bifidobacteria; carbohydrate metabolism; fiber; glycosyl hydrolase; plant glycans; plant oligosaccharides.
Copyright © 2021 Kelly, Munoz-Munoz and van Sinderen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alimentarius C. (2010). Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended 2010. Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission. Rome: FAO.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

