Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment
- PMID: 33613483
- PMCID: PMC7889510
- DOI: 10.3389/fmicb.2021.612675
Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment
Abstract
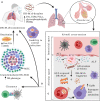
In the last two decades, multi (MDR), extensively (XDR), extremely (XXDR) and total (TDR) drug-resistant Mycobacterium tuberculosis (M.tb) strains have emerged as a threat to public health worldwide, stressing the need to develop new tuberculosis (TB) prevention and treatment strategies. It is estimated that in the next 35 years, drug-resistant TB will kill around 75 million people and cost the global economy $16.7 trillion. Indeed, the COVID-19 pandemic alone may contribute with the development of 6.3 million new TB cases due to lack of resources and enforced confinement in TB endemic areas. Evolution of drug-resistant M.tb depends on numerous factors, such as bacterial fitness, strain's genetic background and its capacity to adapt to the surrounding environment, as well as host-specific and environmental factors. Whole-genome transcriptomics and genome-wide association studies in recent years have shed some insights into the complexity of M.tb drug resistance and have provided a better understanding of its underlying molecular mechanisms. In this review, we will discuss M.tb phenotypic and genotypic changes driving resistance, including changes in cell envelope components, as well as recently described intrinsic and extrinsic factors promoting resistance emergence and transmission. We will further explore how drug-resistant M.tb adapts differently than drug-susceptible strains to the lung environment at the cellular level, modulating M.tb-host interactions and disease outcome, and novel next generation sequencing (NGS) strategies to study drug-resistant TB.
Keywords: Mycobacterium tuberculosis; bacterial–host interactions; drug resistance; evolution; next generation sequencing.
Copyright © 2021 Allué-Guardia, García and Torrelles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arcos J., Sasindran S. J., Moliva J. I., Scordo J. M., Sidiki S., Guo H., et al. (2017). Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner. Mucosal Immunol. 10 1248–1258. 10.1038/mi.2016.115 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

