Collagen Binding Proteins of Gram-Positive Pathogens
- PMID: 33613497
- PMCID: PMC7893114
- DOI: 10.3389/fmicb.2021.628798
Collagen Binding Proteins of Gram-Positive Pathogens
Abstract
Collagens are the primary structural components of mammalian extracellular matrices. In addition, collagens regulate tissue development, regeneration and host defense through interaction with specific cellular receptors. Their unique triple helix structure, which requires a glycine residue every third amino acid, is the defining structural feature of collagens. There are 28 genetically distinct collagens in humans. In addition, several other unrelated human proteins contain a collagen domain. Gram-positive bacteria of the genera Staphylococcus, Streptococcus, Enterococcus, and Bacillus express cell surface proteins that bind to collagen. These proteins of Gram-positive pathogens are modular proteins that can be classified into different structural families. This review will focus on the different structural families of collagen binding proteins of Gram-positive pathogen. We will describe how these proteins interact with the triple helix in collagens and other host proteins containing a collagenous domain and discuss how these interactions can contribute to the pathogenic processes.
Keywords: Gram-positive bacteria; collagen; collagen binding proteins; collagen-like proteins; surface proteins.
Copyright © 2021 Arora, Gordon and Hook.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

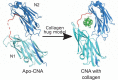
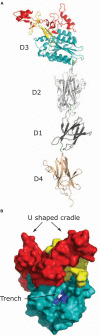

References
-
- Abranches J., Miller J. H., Martinez A. R., Simpson-Haidaris P. J., Burne R. A., Lemos J. A. (2011). The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun. 79 2277–2284. 10.1128/iai.00767-10 - DOI - PMC - PubMed
-
- Arvanitidis A., Karsdal M. A. (2016). “Type XV Collagen,” in Biochemistry of Collagens, Laminins and Elastin, Chap. 15, ed. Karsdal M. A. (Cambridge, MA: Academic Press; ), 97–99.
-
- Bager C. L., Karsdal M. A. (2016). “Type XVIII Collagen,” in Biochemistry of Collagens, Laminins and Elastin, Chap. 18, ed. Karsdal M. A. (Cambridge, MA: Academic Press; ), 113–121.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

