Vitamin B6 Acquisition and Metabolism in Schistosoma mansoni
- PMID: 33613557
- PMCID: PMC7891054
- DOI: 10.3389/fimmu.2020.622162
Vitamin B6 Acquisition and Metabolism in Schistosoma mansoni
Abstract
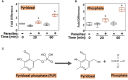
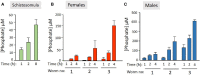
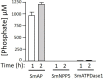
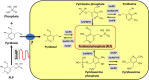
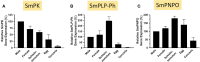
Schistosomes are parasitic platyhelminths that currently infect >200 million people globally. The adult worms can live within the vasculature of their hosts for many years where they acquire all nutrients necessary for their survival and growth. In this work we focus on how Schistosoma mansoni parasites acquire and metabolize vitamin B6, whose active form is pyridoxal phosphate (PLP). We show here that live intravascular stage parasites (schistosomula and adult males and females) can cleave exogenous PLP to liberate pyridoxal. Of the three characterized nucleotide-metabolizing ectoenzymes expressed at the schistosome surface (SmAP, SmNPP5, and SmATPDase1), only SmAP hydrolyzes PLP. Heat-inactivated recombinant SmAP can no longer cleave PLP. Further, parasites whose SmAP gene has been suppressed by RNAi are significantly impaired in their ability to cleave PLP compared to controls. When schistosomes are incubated in murine plasma, they alter its metabolomic profile-the levels of both pyridoxal and phosphate increase over time, a finding consistent with the action of host-exposed SmAP acting on PLP. We hypothesize that SmAP-mediated dephosphorylation of PLP generates a pool of pyridoxal around the worms that can be conveniently taken in by the parasites to participate in essential, vitamin B6-driven metabolism. In addition, since host PLP-dependent enzymes play active roles in inflammatory processes, parasite-mediated cleavage of this metabolite may serve to limit parasite-damaging inflammation. In this work we also identified schistosome homologs of enzymes that are involved in intracellular vitamin B6 metabolism. These are pyridoxal kinase (SmPK) as well as pyridoxal phosphate phosphatase (SmPLP-Ph) and pyridox(am)ine 5'-phosphate oxidase (SmPNPO) and cDNAs encoding these three enzymes were cloned and sequenced. The three genes encoding these enzymes all display high relative expression in schistosomula and adult worms suggestive of robust vitamin B6 metabolism in the intravascular life stages.
Keywords: PLP; alkaline phosphatase; ectoenzyme; parasite; pyridoxal phosphate; schistosome.
Copyright © 2021 Da’dara, Elzoheiry, El-Beshbishi and Skelly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Metabolism of FAD, FMN and riboflavin (vitamin B2) in the human parasitic blood fluke Schistosoma mansoni.bioRxiv [Preprint]. 2024 Mar 12:2024.03.12.584659. doi: 10.1101/2024.03.12.584659. bioRxiv. 2024. Update in: BMC Infect Dis. 2024 Jun 26;24(1):636. doi: 10.1186/s12879-024-09538-z. PMID: 38558993 Free PMC article. Updated. Preprint.
-
Metabolism of FAD, FMN and riboflavin (vitamin B2) in the human parasitic blood fluke Schistosoma mansoni.BMC Infect Dis. 2024 Jun 26;24(1):636. doi: 10.1186/s12879-024-09538-z. BMC Infect Dis. 2024. PMID: 38918706 Free PMC article.
-
Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase.Front Immunol. 2018 Jul 30;9:1746. doi: 10.3389/fimmu.2018.01746. eCollection 2018. Front Immunol. 2018. PMID: 30105025 Free PMC article.
-
Pyridoxal 5'-Phosphate Biosynthesis by Pyridox-(am)-ine 5'-Phosphate Oxidase: Species-Specific Features.Int J Mol Sci. 2024 Mar 9;25(6):3174. doi: 10.3390/ijms25063174. Int J Mol Sci. 2024. PMID: 38542149 Free PMC article. Review.
-
Disorders affecting vitamin B6 metabolism.J Inherit Metab Dis. 2019 Jul;42(4):629-646. doi: 10.1002/jimd.12060. Epub 2019 Mar 20. J Inherit Metab Dis. 2019. PMID: 30671974 Review.
Cited by
-
Excretory/Secretory Proteome of Females and Males of the Hookworm Ancylostoma ceylanicum.Pathogens. 2023 Jan 6;12(1):95. doi: 10.3390/pathogens12010095. Pathogens. 2023. PMID: 36678443 Free PMC article.
-
Schistosoma mansoni and the purinergic halo.Trends Parasitol. 2022 Dec;38(12):1080-1088. doi: 10.1016/j.pt.2022.09.001. Epub 2022 Sep 28. Trends Parasitol. 2022. PMID: 36182536 Free PMC article. Review.
-
Metabolism of FAD, FMN and riboflavin (vitamin B2) in the human parasitic blood fluke Schistosoma mansoni.bioRxiv [Preprint]. 2024 Mar 12:2024.03.12.584659. doi: 10.1101/2024.03.12.584659. bioRxiv. 2024. Update in: BMC Infect Dis. 2024 Jun 26;24(1):636. doi: 10.1186/s12879-024-09538-z. PMID: 38558993 Free PMC article. Updated. Preprint.
-
The riboflavin (vitamin B2) transporter protein (SmaRT) of the human intravascular parasitic trematode Schistosoma mansoni.Heliyon. 2024 Mar 22;10(7):e28271. doi: 10.1016/j.heliyon.2024.e28271. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38601580 Free PMC article.
-
Metabolism of FAD, FMN and riboflavin (vitamin B2) in the human parasitic blood fluke Schistosoma mansoni.BMC Infect Dis. 2024 Jun 26;24(1):636. doi: 10.1186/s12879-024-09538-z. BMC Infect Dis. 2024. PMID: 38918706 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

