The Role of the Z-DNA Binding Domain in Innate Immunity and Stress Granules
- PMID: 33613567
- PMCID: PMC7886975
- DOI: 10.3389/fimmu.2020.625504
The Role of the Z-DNA Binding Domain in Innate Immunity and Stress Granules
Abstract
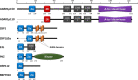
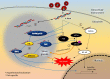
Both DNA and RNA can maintain left-handed double helical Z-conformation under physiological condition, but only when stabilized by Z-DNA binding domain (ZDBD). After initial discovery in RNA editing enzyme ADAR1, ZDBD has also been described in pathogen-sensing proteins ZBP1 and PKZ in host, as well as virulence proteins E3L and ORF112 in viruses. The host-virus antagonism immediately highlights the importance of ZDBD in antiviral innate immunity. Furthermore, Z-RNA binding has been shown to be responsible for the localization of these ZDBD-containing proteins to cytoplasmic stress granules that play central role in coordinating cellular response to stresses. This review sought to consolidate current understanding of Z-RNA sensing in innate immunity and implore possible roles of Z-RNA binding within cytoplasmic stress granules.
Keywords: ADAR1; E3L; PKZ; Z-DNA binding domain; Z-RNA; ZBP1; innate immunity; stress granules.
Copyright © 2021 Chiang, Li and Ng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Novel Z-DNA binding domains in giant viruses.J Biol Chem. 2024 Aug;300(8):107504. doi: 10.1016/j.jbc.2024.107504. Epub 2024 Jun 27. J Biol Chem. 2024. PMID: 38944123 Free PMC article.
-
Proteins that contain a functional Z-DNA-binding domain localize to cytoplasmic stress granules.Nucleic Acids Res. 2013 Nov;41(21):9786-99. doi: 10.1093/nar/gkt750. Epub 2013 Aug 27. Nucleic Acids Res. 2013. PMID: 23982513 Free PMC article.
-
How Z-DNA/RNA binding proteins shape homeostasis, inflammation, and immunity.BMB Rep. 2020 Sep;53(9):453-457. doi: 10.5483/BMBRep.2020.53.9.141. BMB Rep. 2020. PMID: 32731914 Free PMC article. Review.
-
The Structure of the Cyprinid herpesvirus 3 ORF112-Zα·Z-DNA Complex Reveals a Mechanism of Nucleic Acids Recognition Conserved with E3L, a Poxvirus Inhibitor of Interferon Response.J Biol Chem. 2015 Dec 25;290(52):30713-25. doi: 10.1074/jbc.M115.679407. Epub 2015 Nov 11. J Biol Chem. 2015. PMID: 26559969 Free PMC article.
-
Zalpha-domains: at the intersection between RNA editing and innate immunity.Semin Cell Dev Biol. 2012 May;23(3):275-80. doi: 10.1016/j.semcdb.2011.11.001. Epub 2011 Nov 7. Semin Cell Dev Biol. 2012. PMID: 22085847 Review.
Cited by
-
A Wonderful Journey: The Diverse Roles of Adenosine Deaminase Action on RNA 1 (ADAR1) in Central Nervous System Diseases.CNS Neurosci Ther. 2025 Jan;31(1):e70208. doi: 10.1111/cns.70208. CNS Neurosci Ther. 2025. PMID: 39753993 Free PMC article. Review.
-
ADARs: pleiotropy in function, versatility in application.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf672. doi: 10.1093/nar/gkaf672. Nucleic Acids Res. 2025. PMID: 40671518 Free PMC article. Review.
-
Susceptibility and Permissivity of Zebrafish (Danio rerio) Larvae to Cypriniviruses.Viruses. 2023 Mar 17;15(3):768. doi: 10.3390/v15030768. Viruses. 2023. PMID: 36992477 Free PMC article.
-
Adoption of A-Z Junctions in RNAs by Binding of Zα Domains.Methods Mol Biol. 2023;2651:251-275. doi: 10.1007/978-1-0716-3084-6_18. Methods Mol Biol. 2023. PMID: 36892773 Free PMC article.
-
Searching for New Z-DNA/Z-RNA Binding Proteins Based on Structural Similarity to Experimentally Validated Zα Domain.Int J Mol Sci. 2022 Jan 11;23(2):768. doi: 10.3390/ijms23020768. Int J Mol Sci. 2022. PMID: 35054954 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

