Next Generation Sequencing Based Non-invasive Prenatal Testing (NIPT): First Report From Saudi Arabia
- PMID: 33613643
- PMCID: PMC7889598
- DOI: 10.3389/fgene.2021.630787
Next Generation Sequencing Based Non-invasive Prenatal Testing (NIPT): First Report From Saudi Arabia
Abstract
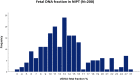
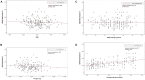
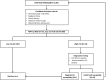
Background: Non-invasive prenatal testing (NIPT) for aneuploidy in pregnant women screening has been recently established in Saudi Arabia. We aim from this study to report our experience in the implementation of this new technology in clinical practice and to assess factors influencing cell-free fetal (cffDNA) fraction and successful NIPT reporting. Methods: In total, 200 pregnant women were subjected to the NIPT test using standard methods. Next-generation sequencing (NGS) was used to analyze cffDNA in maternal plasma. Results: Out of the 200 NIPT cases, the average age of pregnant women was 35 ± 6 years (range: 21-48 years). The average cffDNA fraction of reported cases was 13.72% (range: 3-31%). Out of these 200 cases, 187 (93.5%) were at low risk, while 13 (6.5%) cases revealed high risk for aneuploidy. Among these chromosomal abnormalities, 7 (3.5%) cases of Down's syndrome, 5 (2.5%) Edwards' Syndrome, and only 1 case of (0.5%) Patau's syndrome was observed. Out of the 13 high-risk cases, 2 (15.3%) were found in women below the age of 30. Conclusion: This is the first study reporting the successful implementation of an in-house NIPT screening service in Saudi Arabia. Our data showed high accuracy and sensitivity to detect high-risk cases indicating the usefulness of such a technique as an alternative to invasive testing and (hopefully) will change the common screening practice for pregnant women in Saudi Arabia.
Keywords: aneuploidy; chromosomal duplications; deletions; fetal DNA; next generation sequencing; non-invasive prenatal testing (NIPT).
Copyright © 2021 Alyafee, Al Tuwaijri, Alam, Umair, Haddad, Alharbi, Ballow, Al Drees, AlAbdulrahman, Al Khaldi and Alfadhel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

