Recombinant immunotoxin targeting GPC3 is cytotoxic to H446 small cell lung cancer cells
- PMID: 33613711
- PMCID: PMC7859473
- DOI: 10.3892/ol.2021.12483
Recombinant immunotoxin targeting GPC3 is cytotoxic to H446 small cell lung cancer cells
Abstract
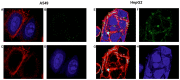
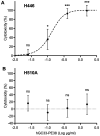
Glypican-3 (GPC3) is a cell membrane glycoprotein that regulates cell growth and proliferation. Aberrant expression or distribution of GPC3 underlies developmental abnormalities and the development of solid tumours. The strongest evidence for the participation of GPC3 in carcinogenesis stems from studies on hepatocellular carcinoma and lung squamous cell carcinoma. To the best of our knowledge, the role of the GPC3 protein and its potential therapeutic application have never been studied in small cell lung carcinoma (SCLC), despite the known involvement of associated pathways and the high mortality caused by this disease. Therefore, the aim of the present study was to examine GPC3 targeting for SCLC immunotherapy. An immunotoxin carrying an anti-GPC3 antibody (hGC33) and Pseudomonas aeruginosa exotoxin A 38 (PE38) was generated. This hGC33-PE38 protein was overexpressed in E. coli and purified. ADP-ribosylation activity was tested in vitro against eukaryotic translation elongation factor 2. Cell internalisation ability was confirmed by confocal microscopy. Cytotoxicity was analysed by treating liver cancer (HepG2, SNU-398 and SNU-449) and lung cancer (NCI-H510A, NCI-H446, A549 and SK-MES1) cell lines with hGC33-PE38 and estimating viable cells number. A BrdU assay was employed to verify anti-proliferative activity of hGC33-PE38 on treated cells. Fluorescence-activated cell sorting was used for the detection of cell membrane-bound GPC3. The hGC33-PE38 immunotoxin displayed enzymatic activity comparable to native PE38. The protein was efficiently internalised by GPC3-positive cells. Moreover, hGC33-PE38 was cytotoxic to HepG2 cells but had no effect on known GPC3-negative cell lines. The H446 cells were sensitive to hGC33-PE38 (IC50, 70.6±4.6 ng/ml), whereas H510A cells were resistant. Cell surface-bound GPC3 was abundant on the membranes of H446 cells, but absent on H510A. Altogether, the present findings suggested that GPC3 could be considered as a potential therapeutic target for SCLC immunotherapy.
Keywords: Wnt/β-catenin pathway; glypican-3; hepatocellular carcinoma; immunotherapy; immunotoxin; small cell lung carcinoma.
Copyright: © Rodakowska et al.
Figures





Similar articles
-
Engineered E. coli OMVs Carrying the Membrane-Binding hGC33 Fragment Precisely Target Liver Cancer and Effectively Treat Tumor.Int J Nanomedicine. 2025 May 22;20:6573-6590. doi: 10.2147/IJN.S513508. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40433120 Free PMC article.
-
hGC33-Modified and Sorafenib-Loaded Nanoparticles have a Synergistic Anti-Hepatoma Effect by Inhibiting Wnt Signaling Pathway.Nanoscale Res Lett. 2020 Nov 26;15(1):220. doi: 10.1186/s11671-020-03451-5. Nanoscale Res Lett. 2020. PMID: 33242103 Free PMC article.
-
Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma.World J Gastroenterol. 2016 Jan 7;22(1):275-83. doi: 10.3748/wjg.v22.i1.275. World J Gastroenterol. 2016. PMID: 26755876 Free PMC article. Review.
-
Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer.Cancer Res. 2008 Dec 1;68(23):9832-8. doi: 10.1158/0008-5472.CAN-08-1973. Cancer Res. 2008. PMID: 19047163
-
Glypican-3 Targeting Immunotoxins for the Treatment of Liver Cancer.Toxins (Basel). 2016 Sep 22;8(10):274. doi: 10.3390/toxins8100274. Toxins (Basel). 2016. PMID: 27669301 Free PMC article. Review.
Cited by
-
Comparison of two immunotoxins against DLL3 receptor; as an inhibitor for small cell lung cancer.Front Mol Biosci. 2025 Mar 19;12:1506768. doi: 10.3389/fmolb.2025.1506768. eCollection 2025. Front Mol Biosci. 2025. PMID: 40177519 Free PMC article.
-
Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives.Front Oncol. 2022 Apr 28;12:891187. doi: 10.3389/fonc.2022.891187. eCollection 2022. Front Oncol. 2022. PMID: 35574361 Free PMC article. Review.
-
Design of two immunotoxins based rovalpituzumab antibody against DLL3 receptor; a promising potential opportunity.Res Pharm Sci. 2022 Jul 14;17(4):428-444. doi: 10.4103/1735-5362.350243. eCollection 2022 Aug. Res Pharm Sci. 2022. PMID: 36034078 Free PMC article.
References
-
- De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–635. doi: 10.1083/jcb.200302152. - DOI - PMC - PubMed
-
- Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, Puricelli L, de Kier Joffé EB. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol. 2008;23:1333–1340. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
