Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs
- PMID: 33613731
- PMCID: PMC7878464
- DOI: 10.1016/j.ajps.2020.05.003
Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs
Abstract
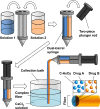
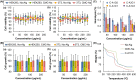
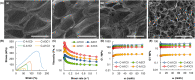
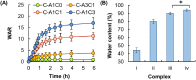
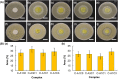
Hydrogels are soft materials consisting of a three-dimensional network of polymer chains. Over the years, hydrogels with different compositions have been developed as drug carriers for diverse biomedical applications, ranging from cancer therapy and wound care to the treatment of neurodegenerative and inflammatory diseases. Most of these carriers, however, are designed only to deliver single agents. Carriers based on hydrogels for co-delivery of multiple agents, with the release rate of each of the co-delivered agents tunable, are lacking. This study reports a one-pot method of fabricating alginate-based complex fibers with the Janus morphology, with carboxymethyl cellulose sodium functioning as a polymeric modifier of the properties of each of the fiber compartments. By using malachite green and minocycline hydrochloride as model drugs, the generated fibers demonstrate the capacity of enabling the release profile of each of the co-delivered drugs to be precisely controlled. Along with their negligible toxicity and the retention of the activity of the loaded drugs, the complex fibers reported in this study warrant further development and optimization for applications that involve co-delivery of multiple agents.
Keywords: Co-delivery; Complex fiber; Controlled release; Janus morphology; Tunable release profiles.
© 2020 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures

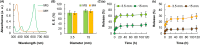

References
-
- Norouzi M., Nazari B., Miller D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21:1835–1849. - PubMed
-
- Henderson P.W., Singh S.P., Krijgh D.D., Yamamoto M., Rafii D.C., Sung J.J. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regen. 2011;19:420–425. - PubMed
-
- Ren Y., Zhao X., Liang X., Ma P.X., Guo B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson's disease. Int J Biol Macromol. 2017;105:1079–1087. - PubMed
-
- Lai W.F., Susha A.S., Rogach A.L. Multicompartment microgel beads for co-delivery of multiple drugs at individual release rates. ACS Appl Mater Interfaces. 2016;8:871–880. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

