Androgen Plays a Carcinogenic Role in EOC via the PI3K/AKT Signaling Pathway in an AR-Dependent Manner
- PMID: 33613770
- PMCID: PMC7890324
- DOI: 10.7150/jca.51099
Androgen Plays a Carcinogenic Role in EOC via the PI3K/AKT Signaling Pathway in an AR-Dependent Manner
Abstract
Background: Epithelial ovarian cancer (EOC) is one of the most common gynecological cancers with the highest mortality rate. Studies indicate that androgens contribute to initiation or progression of EOC through poorly understood mechanisms, however, in the phase II clinical studies of antiandrogen therapy for EOC, neither flutamide nor bicalutamide showed good antitumor effects. Based on the contradictions, the purpose of this study was to explore the role of androgen receptor (AR) in the androgen pathogenesis of EOC and the possible mechanism, and further to find an indicator to screen the anti-androgen therapy sensitive cases. Methods: In this study, 70 EOC biopsies and 17 para-cancerous tissues with complete medical information were collected and analyzed. The expression of the androgen receptor (AR) was detected by immunohistochemistry. In addition, ovarian cancer cell lines were used for in vitro studies to further explore the role of androgen in cell proliferation and the possible mechanisms. Results: The results showed that the expression of AR in ovarian cancer tissues was significantly elevated compared to the para-cancerous tissues, particularly in low-grade EOC, and the presence of high AR expression often suggested a worse prognosis. The in vitro study indicated that testosterone promoted the proliferation of the AR-positive SKOV3 cell line, which could be blocked by flutamide, but not in the AR-negative A2780 cell line. Next, we showed that testosterone-promoted proliferation in SKOV3 cells was abolished after we knocked out the AR. The mechanism studies revealed that the p-AKT expression in the ovarian cancer tissue was increased compared to the para-cancerous tissues, following a pattern similar to the increase of AR expression. Furthermore, the deletion and overexpression of SKOV3 cells' ARs lead to corresponding changes in the p-AKT levels. In addition, the BEZ235, an inhibitor of the PI3K/AKT signaling pathway blocked the proliferative effect of testosterone in SKOV3 cells. Conclusion: We showed that testosterone was able to promote the proliferation of ovarian cancer cells through activating the PI3K/AKT signaling pathway in an AR dependent manner and AR may be a screening indicator for anti-androgen therapy sensitive cases of EOC.
Keywords: PI3K/AKT signalling pathway; androgen receptor (AR); ovarian carcinoma; proliferation promotion; tissue microarray (TMA).
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

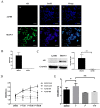

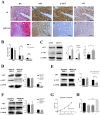
 ± S) and the analysis of the numeric data relied on t-test. (C) Western blotting analysis of AKT and p-AKT was performed in the SKOV3 and A2780 cell lines. Protein levels of AKT and p-AKT were detected as bands with a molecular mass of 60 kDa. (D, E and F) Expression of AKT and p-AKT in the cells after the knockout and overexpression of AR. (G) BEZ235 concentration vs inhibition rate curve (IC50=187 nM), and (H) BEZ235 blocked the proliferative effects of testosterone in the SKOV3 cell line (p<0.001).
± S) and the analysis of the numeric data relied on t-test. (C) Western blotting analysis of AKT and p-AKT was performed in the SKOV3 and A2780 cell lines. Protein levels of AKT and p-AKT were detected as bands with a molecular mass of 60 kDa. (D, E and F) Expression of AKT and p-AKT in the cells after the knockout and overexpression of AR. (G) BEZ235 concentration vs inhibition rate curve (IC50=187 nM), and (H) BEZ235 blocked the proliferative effects of testosterone in the SKOV3 cell line (p<0.001).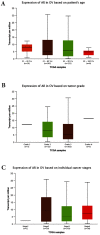
Similar articles
-
Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer.Gynecol Oncol. 2012 Jan;124(1):142-7. doi: 10.1016/j.ygyno.2011.09.004. Epub 2011 Oct 15. Gynecol Oncol. 2012. PMID: 22001143
-
Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression.BMC Cancer. 2019 Jun 24;19(1):618. doi: 10.1186/s12885-019-5824-9. BMC Cancer. 2019. PMID: 31234823 Free PMC article.
-
Antiandrogen Flutamide-Induced Restoration of miR-449 Expression Mitigates Functional Biomarkers Associated with Ovarian Cancer Risk.medRxiv [Preprint]. 2024 Feb 29:2024.02.26.24303311. doi: 10.1101/2024.02.26.24303311. medRxiv. 2024. Update in: Sci Rep. 2024 Dec 2;14(1):29937. doi: 10.1038/s41598-024-80173-z. PMID: 38464045 Free PMC article. Updated. Preprint.
-
Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials.Int J Mol Sci. 2021 Jul 20;22(14):7748. doi: 10.3390/ijms22147748. Int J Mol Sci. 2021. PMID: 34299364 Free PMC article. Review.
-
Molecular pathways in reproductive cancers: a focus on prostate and ovarian cancer.Cancer Cell Int. 2025 Feb 3;25(1):33. doi: 10.1186/s12935-025-03658-5. Cancer Cell Int. 2025. PMID: 39901204 Free PMC article. Review.
Cited by
-
Diane-35 and metformin therapy in rats with endometrial lesions induced by dihydrotestosterone exposure.Ann Transl Med. 2023 Mar 31;11(6):247. doi: 10.21037/atm-21-2441. Epub 2023 Feb 2. Ann Transl Med. 2023. PMID: 37082665 Free PMC article.
-
Screening and Identification of a Prognostic Model of Ovarian Cancer by Combination of Transcriptomic and Proteomic Data.Biomolecules. 2023 Apr 18;13(4):685. doi: 10.3390/biom13040685. Biomolecules. 2023. PMID: 37189432 Free PMC article.
-
Testosterone, β-estradiol, and hepatocellular carcinoma: stimulation or inhibition? A comparative effect analysis on cell cycle, apoptosis, and Wnt signaling of HepG2 cells.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):6121-6133. doi: 10.1007/s00210-024-03019-5. Epub 2024 Feb 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38421409
References
-
- Alharbi M, Zuniga F, Elfeky O. et al. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr Relat Cancer. 2018;25:R663–R85. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Jin Y, Feng SJ, Qiu S. et al. LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med Pharmacol Sci. 2017;21:3176–84. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

