Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF- κ B- and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production
- PMID: 33613822
- PMCID: PMC7878077
- DOI: 10.1155/2021/1246491
Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF- κ B- and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production
Abstract
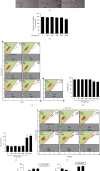
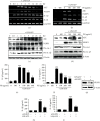
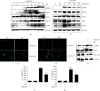
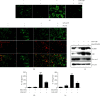
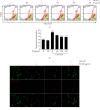
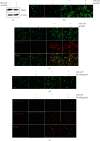
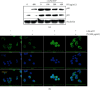
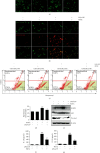
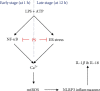
Anthocyanins from the petals of Hibiscus syriacus L. (PS) possess anti-inflammatory, antioxidant, and antimelanogenic activities. However, it remains unclear whether PS inhibit the NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation and assembly. This study is aimed at investigating whether PS downregulate NLRP3-mediated inflammasome by inhibiting nuclear factor-κB (NF-κB) and endoplasmic reticulum (ER) stress. BV2 microglia cells were treated with PS in the presence of lipopolysaccharide and adenosine triphosphate (LPS/ATP), and the NLRP3-related signaling pathway was investigated. In this study, we found that LPS/ATP treatment activated the NLRP3 inflammasome, which resulted in the release of interleukin-1β (IL-1β) and IL-18. Meanwhile, PS reduced LPS/ATP-mediated NLRP3 inflammasome at 12 h by inhibiting ER stress-mediated Ca2+ accumulation and subsequent mitochondrial reactive oxygen species (mtROS) production, which, in turn, decreased IL-1β and IL-18 release. Furthermore, PS inhibited the NLRP3 inflammasome 1 h after LPS/ATP treatment by suppressing the NF-κB pathway, which downregulated Ca2+ accumulation and mtROS production. These data showed that PS negatively regulated activation of the NLRP3 inflammasome in a time-different manner by inhibiting the NF-κB signaling pathway in the early stage and the ER stress response in the late stage. The pathways shared Ca2+ accumulation-mediated mtROS production, which was significantly inhibited in the presence of PS. In conclusion, our results suggested that PS has potential as a supplement against NLRP3 inflammasome-related inflammatory disorders; nevertheless, further studies are needed to determine the effect of PS in the noncanonical NLRP3 inflammasome pathways and pathological conditions in vivo.
Copyright © 2021 Ilandarage Menu Neelaka Molagoda et al.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Benzyl isothiocyanate inhibits inflammasome activation in E. coli LPS-stimulated BV2 cells.Int J Mol Med. 2016 Sep;38(3):912-8. doi: 10.3892/ijmm.2016.2667. Epub 2016 Jul 6. Int J Mol Med. 2016. PMID: 27430883
-
HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production.J Hepatol. 2017 Apr;66(4):693-702. doi: 10.1016/j.jhep.2016.12.018. Epub 2016 Dec 24. J Hepatol. 2017. PMID: 28027970
-
Crosstalk between ER stress, NLRP3 inflammasome, and inflammation.Appl Microbiol Biotechnol. 2020 Jul;104(14):6129-6140. doi: 10.1007/s00253-020-10614-y. Epub 2020 May 24. Appl Microbiol Biotechnol. 2020. PMID: 32447438 Review.
-
Possible protective effect of resolvin D1 on inflammation in atrial fibrillation: involvement of ER stress mediated the NLRP3 inflammasome pathway.Naunyn Schmiedebergs Arch Pharmacol. 2021 Aug;394(8):1613-1619. doi: 10.1007/s00210-021-02115-0. Epub 2021 Jul 3. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 34216224 Review.
Cited by
-
Transcriptome and proteome profile of jejunum in chickens challenged with Salmonella Typhimurium revealed the effects of dietary bilberry anthocyanin on immune function.Front Microbiol. 2023 Nov 20;14:1266977. doi: 10.3389/fmicb.2023.1266977. eCollection 2023. Front Microbiol. 2023. PMID: 38053560 Free PMC article.
-
NMDA receptor blockade attenuates Japanese encephalitis virus infection-induced microglia activation.J Neuroinflammation. 2024 Nov 7;21(1):291. doi: 10.1186/s12974-024-03288-0. J Neuroinflammation. 2024. PMID: 39511597 Free PMC article.
-
A Levee to the Flood: Pre-injury Neuroinflammation and Immune Stress Influence Traumatic Brain Injury Outcome.Front Aging Neurosci. 2022 Jan 12;13:788055. doi: 10.3389/fnagi.2021.788055. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35095471 Free PMC article. Review.
-
Molecular Mechanisms of Inflammasome in Ischemic Stroke Pathogenesis.Pharmaceuticals (Basel). 2022 Sep 21;15(10):1168. doi: 10.3390/ph15101168. Pharmaceuticals (Basel). 2022. PMID: 36297283 Free PMC article. Review.
-
Role of NLRP3 Inflammasome in Myocardial Ischemia-Reperfusion Injury and Ventricular Remodeling.Med Sci Monit. 2022 Jan 19;28:e934255. doi: 10.12659/MSM.934255. Med Sci Monit. 2022. PMID: 35042840 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

