Integrated metabolomics and network pharmacology to reveal the mechanisms of hydroxysafflor yellow A against acute traumatic brain injury
- PMID: 33613866
- PMCID: PMC7868816
- DOI: 10.1016/j.csbj.2021.01.033
Integrated metabolomics and network pharmacology to reveal the mechanisms of hydroxysafflor yellow A against acute traumatic brain injury
Abstract
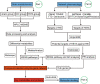
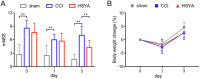
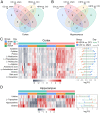
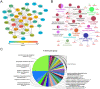
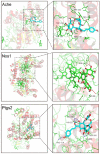
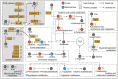
Traumatic brain injury (TBI) has become a leading cause of mortality, morbidity and disability worldwide. Hydroxysafflor yellow A (HSYA) is effective in treating TBI, but the potential mechanisms require further exploration. We aimed to reveal the mechanisms of HSYA against acute TBI by an integrated strategy combining metabolomics with network pharmacology. A controlled cortical impact (CCI) rat model was established, and neurological functions were evaluated. Metabolomics of brain tissues was used to identify differential metabolites, and the metabolic pathways were enriched by MetaboAnalyst. Then, network pharmacology was applied to dig out the potential targets against TBI induced by HSYA. The integrated network of metabolomics and network pharmacology was constructed based on Cytoscape. Finally, the obtained key targets were verified by molecular docking. HSYA alleviated the neurological deficits of TBI. Fifteen potentially significant metabolites were found to be involved in the therapeutic effects of HSYA against acute TBI. Most of these metabolites were regulated to recover after HSYA treatment. We found 10 hub genes according to network pharmacology, which was partly consistent with the metabolomics findings. Further integrated analysis focused on 4 key targets, including NOS1, ACHE, PTGS2 and XDH, as well as their related core metabolites and pathways. Molecular docking showed high affinities between key targets and HSYA. Region-specific metabolic alterations in the cortex and hippocampus were illuminated. This study reveals the complicated mechanisms of HSYA against acute TBI. Our work provides a novel paradigm to identify the potential mechanisms of pharmacological effects derived from a natural compound.
Keywords: Hydroxysafflor yellow A; Mechanisms; Metabolomics; Network pharmacology; Traumatic brain injury.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. - PubMed
-
- Chen L., Xiang Y., Kong L., Zhang X., Sun B., Wei X. Hydroxysafflor yellow A protects against cerebral ischemia-reperfusion injury by anti-apoptotic effect through PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 2013;38(11):2268–2275. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
