Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: in vitro experiments and theoretical modelling study
- PMID: 33614097
- PMCID: PMC7890514
- DOI: 10.1098/rsos.201785
Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: in vitro experiments and theoretical modelling study
Abstract
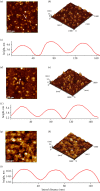
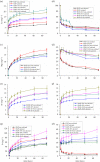
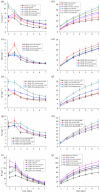
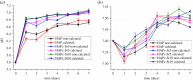
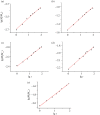
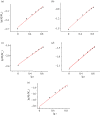
Multi-substituted hydroxyapatites (ms-HAPs) are currently gaining more consideration owing to their multifunctional properties and biomimetic structure, owning thus an enhanced biological potential in orthopaedic and dental applications. In this study, nano-hydroxyapatite (HAP) substituted with multiple cations (Sr2+, Mg2+ and Zn2+) for Ca2+ and anion ( ) for and OH-, specifically HAPc-5%Sr and HAPc-10%Sr (where HAPc is HAP-1.5%Mg-0.2%Zn-0.2%Si), both lyophilized non-calcined and lyophilized calcined, were evaluated for their in vitro ions release. These nanomaterials were characterized by scanning electron microscopy, field emission-scanning electron microscopy and energy-dispersive X-ray, as well as by atomic force microscope images and by surface specific areas and porosity. Further, the release of cations and of phosphate anions were assessed from nano-HAP and ms-HAPs, both in water and in simulated body fluid, in static and simulated dynamic conditions, using inductively coupled plasma optical emission spectrometry. The release profiles were analysed and the influence of experimental conditions was determined for each of the six nanomaterials and for various periods of time. The pH of the samples soaked in the immersion liquids was also measured. The ion release mechanism was theoretically investigated using the Korsmeyer-Peppas model. The results indicated a mechanism principally based on diffusion and dissolution, with possible contribution of ion exchange. The surface of ms-HAP nanoparticles is more susceptible to dissolution into immersion liquids owing to the lattice strain provoked by simultaneous multi-substitution in HAP structure. According to the findings, it is rational to suggest that both materials HAPc-5%Sr and HAPc-10%Sr are bioactive and can be potential candidates in bone tissue regeneration.
Keywords: Ca; Korsmeyer–Peppas model; Mg and P release; Sr; multi-substituted hydroxyapatites; release mechanism.
© 2021 The Authors.
Conflict of interest statement
we declare we have no competing interests.
Figures


References
-
- Oonishi H Jr, Oonishi H, Ohashi H, Kawahara I, Hanaoka Y, Iwata R, Hench LL. 2014. Chapter 2. In Advances in calcium phosphate biomaterials, vol. 2 (ed. Ben-Nissan B), pp. 19–49. Berlin, Germany: Springer.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
