A Multimodal Hair-Loss Treatment Strategy Using a New Topical Phytoactive Formulation: A Report of Five Cases
- PMID: 33614172
- PMCID: PMC7878086
- DOI: 10.1155/2021/6659943
A Multimodal Hair-Loss Treatment Strategy Using a New Topical Phytoactive Formulation: A Report of Five Cases
Abstract
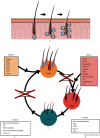
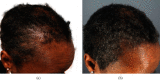
Introduction. Current approved medications for hair loss, such as topical minoxidil and oral finasteride, may have suboptimal efficacy or side effects precluding continued use in some patients. Thus, we report an evaluation of the efficacy, safety, and tolerability of a new topical botanical formulation -GASHEE containing over 12 phytoactive ingredients that affect multiple targets in the cascade of pathophysiologic events that cause hair loss. Five patients with various hair-loss conditions, including cases of previous treatment failures, are presented. Case Presentation. This is a case series of four women and one man with hair loss due to various causes, four of whom had failed minoxidil treatment for over a year. All patients used the topical treatment as a sole therapy for at least 3 months before the documentation of outcomes, which involved interval changes noted through each patient's account, direct observation, and photography. Discussion. In all patients, we observed significant improvements in hair regrowth in the nape, crown, vertex, and temple areas after 3-15 months of treatment. All patients were highly satisfied with their results and reported no adverse events. Although the use of botanicals in the treatment of hair loss is in an infant stage, the new formulation used in this study demonstrated a good efficacy related to hair growth, warranting further evaluation.
Copyright © 2021 Sanusi Umar and Marissa J. Carter.
Conflict of interest statement
Dr. Sanusi Umar has ownership shares in FineTouch Laboratories, Inc., which owns Dr. UGro Gashee®. Dr Marissa J. Carter has no conflicts of interest.
Figures







Similar articles
-
Lichen Planopilaris Responsive to a Novel Phytoactive Botanical Treatment: A Case Series.Dermatol Ther (Heidelb). 2022 Jul;12(7):1697-1710. doi: 10.1007/s13555-022-00749-3. Epub 2022 Jun 8. Dermatol Ther (Heidelb). 2022. PMID: 35674981 Free PMC article.
-
Treatment-Refractory Central Centrifugal Cicatricial Alopecia Responsive to a Novel Botanical Treatment.Clin Cosmet Investig Dermatol. 2022 Apr 8;15:609-619. doi: 10.2147/CCID.S358618. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 35422647 Free PMC article.
-
Clinical Efficacy of a Topical Compounded Formulation in Male Androgenetic Alopecia: Minoxidil 10%, Finasteride 0.1%, Biotin 0.2%, and Caffeine Citrate 0.05% Hydroalcoholic Solution.Int J Pharm Compd. 2020 Jan-Feb;24(1):69-76. Int J Pharm Compd. 2020. PMID: 32023218
-
Androgenetic alopecia: an evidence-based treatment update.Am J Clin Dermatol. 2014 Jul;15(3):217-30. doi: 10.1007/s40257-014-0077-5. Am J Clin Dermatol. 2014. PMID: 24848508 Review.
-
Topical minoxidil therapy for hair regrowth.Clin Pharm. 1987 May;6(5):386-92. Clin Pharm. 1987. PMID: 3311578 Review.
Cited by
-
Mechanistic synergy of hair growth promotion by the Avicennia marina extract and its active constituent (avicequinone C) in dermal papilla cells isolated from androgenic alopecia patients.PLoS One. 2023 Apr 21;18(4):e0284853. doi: 10.1371/journal.pone.0284853. eCollection 2023. PLoS One. 2023. PMID: 37083946 Free PMC article.
-
Evaluation of Herbal Hair Lotion loaded with Rosemary for Possible Hair Growth in C57BL/6 Mice.Adv Biomed Res. 2023 Mar 21;12:60. doi: 10.4103/abr.abr_306_21. eCollection 2023. Adv Biomed Res. 2023. PMID: 37200757 Free PMC article.
-
Technological Advances in Anti-hair Loss and Hair Regrowth Cosmeceuticals: Mechanistic Breakthroughs and Industrial Prospects Driven by Multidisciplinary Collaborative Innovation.Aesthetic Plast Surg. 2025 Aug 11. doi: 10.1007/s00266-025-05077-3. Online ahead of print. Aesthetic Plast Surg. 2025. PMID: 40790388 Review.
-
Lichen Planopilaris Responsive to a Novel Phytoactive Botanical Treatment: A Case Series.Dermatol Ther (Heidelb). 2022 Jul;12(7):1697-1710. doi: 10.1007/s13555-022-00749-3. Epub 2022 Jun 8. Dermatol Ther (Heidelb). 2022. PMID: 35674981 Free PMC article.
-
Treatment-Refractory Central Centrifugal Cicatricial Alopecia Responsive to a Novel Botanical Treatment.Clin Cosmet Investig Dermatol. 2022 Apr 8;15:609-619. doi: 10.2147/CCID.S358618. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 35422647 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

