Dermoscopic Aspects of Cutaneous Adverse Drug Reactions
- PMID: 33614215
- PMCID: PMC7875668
- DOI: 10.5826/dpc.1101a136
Dermoscopic Aspects of Cutaneous Adverse Drug Reactions
Abstract
Background: Little is known about the dermoscopic evaluation of cutaneous adverse drug reactions (CADRs).
Objectives: To evaluate the dermoscopic patterns of CADRs and identify those associated with severe cutaneous adverse reactions to drugs (SCARDs).
Patients and methods: Patients included in this study from May 2015 to April 2016 had presented with CADRs. CADR presentation and classification were based on standard criteria. SCARDs included Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), overlap SJS/TEN, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP). The dermoscopic features of CADRs were described and compared according to the severity of the reactions.
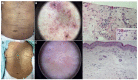
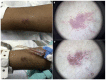
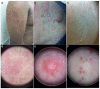
Results: Sixty-nine patients were included. Sixteen patients (23.2%) presented SCARDs. The main dermoscopic findings in SJS, overlap SJS/TEN and TEN were black dots or necrotic areas (100%). Erosion [respectively, 4/6 (66.7%), 3/3 (100%) and 1/1 (100%)], necrotic borders [respectively, 4/6 (66.7%), 3/3 (100%) and 1/1, (100%)] and epidermal detachment [respectively, 5/6 (83.3%); 2/3 (66.7%) and 1/1 (100%)] were also common among these reactions. Erythema and purpuric dots were the main dermoscopic findings [respectively, 5/6 (83.3%) and 4/6 (66.7%)] in DRESS. In non-severe reactions, the most prevalent structures were erythema and purpura in exanthema [respectively, 31/33 (93.9%) and 24/33 (72.7%)] and erythema and vascular structures in urticarial reactions [respectively, 6/6 (100%) and 3/6 (50%)]. Black dots or necrotic areas, epidermal detachment, necrotic borders and erosion were highly associated with SCARDs (P < 0.001).
Conclusions: Dermoscopy improves clinical recognition of SCARDs.
Keywords: cutaneous adverse drug reactions; dermoscopic patterns; dermoscopy; drug eruptions.
©2021 Rossi, et al.
Conflict of interest statement
Competing interests: The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Epidemiology of cutaneous adverse drug reactions.Allergol Select. 2017 Aug 4;1(1):96-108. doi: 10.5414/ALX01508E. eCollection 2017. Allergol Select. 2017. PMID: 30402608 Free PMC article. Review.
-
Clinical features and prognostic factors of severe cutaneous adverse drug reactions: A single-center retrospective study of 209 cases in China.Int Immunopharmacol. 2023 Jan;114:109530. doi: 10.1016/j.intimp.2022.109530. Epub 2022 Dec 9. Int Immunopharmacol. 2023. PMID: 36508915
-
Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist?Orphanet J Rare Dis. 2012 Sep 25;7:72. doi: 10.1186/1750-1172-7-72. Orphanet J Rare Dis. 2012. PMID: 23009177 Free PMC article.
-
A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions.Br J Dermatol. 2013 Mar;168(3):555-62. doi: 10.1111/bjd.12125. Br J Dermatol. 2013. PMID: 23136927
-
Skin tests in the work-up of cutaneous adverse drug reactions: A review and update.Contact Dermatitis. 2022 May;86(5):344-356. doi: 10.1111/cod.14063. Epub 2022 Mar 9. Contact Dermatitis. 2022. PMID: 35122269 Review.
Cited by
-
Utility of Dermoscopy in the Diagnosis of Erythroderma: A Cross-Sectional Study.Indian Dermatol Online J. 2023 Oct 17;14(6):821-828. doi: 10.4103/idoj.idoj_678_22. eCollection 2023 Nov-Dec. Indian Dermatol Online J. 2023. PMID: 38099018 Free PMC article.
-
A Single-Center Cross-Sectional Observational Study on Dermoscopy of Genital Mucosal Disorders in a Tertiary Care Center in Central India.Indian Dermatol Online J. 2024 Oct 28;15(6):955-962. doi: 10.4103/idoj.idoj_868_23. eCollection 2024 Nov-Dec. Indian Dermatol Online J. 2024. PMID: 39640463 Free PMC article.
-
Atypical Presentation of Rapidly Progressive Cutaneous Metastases of Clear Cell Renal Carcinoma: A Case Report.Medicina (Kaunas). 2024 Nov 1;60(11):1797. doi: 10.3390/medicina60111797. Medicina (Kaunas). 2024. PMID: 39596982 Free PMC article. Review.
-
A Single Centre Cross-Sectional Observational Study on Dermatoscopy of Oral Mucosal Disorders and Histopathological Correlation in Tertiary Care Centre from Central India.Indian J Dermatol. 2025 Jul-Aug;70(4):177-187. doi: 10.4103/ijd.ijd_1145_23. Epub 2025 Jun 30. Indian J Dermatol. 2025. PMID: 40740406 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
