Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Literature Review
- PMID: 33614223
- PMCID: PMC7875661
- DOI: 10.5826/dpc.1101a155
Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Literature Review
Abstract
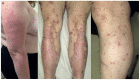
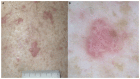
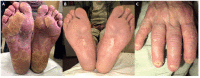
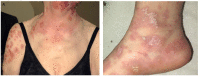
Immune checkpoints assist with self-tolerance and minimize collateral tissue damage when immune responses are activated. Although immune checkpoint inhibitors (CPIs) are characterized by a favorable risk/benefit ratio, immune checkpoint blockade has been associated with a new subset of autoimmune-like toxicities, named immune-related adverse events (irAEs). Dermatologic reactions are among the most prevalent irAEs triggered by CPIs. In a majority of cases they are self-limiting and readily manageable. However, it is not uncommon that they result in severe skin involvement and impairment of patients' quality of life. Awareness of the spectrum of cutaneous irAEs is mandatory for every clinician involved in the management of oncologic patients. The role of the dermatologists is essential because early recognition and appropriate management of skin toxicity may prevent dose modifications and discontinuation of CPIs. The latter is particularly relevant, considering that recent data suggest favorable oncologic response in patients developing irAEs.
Keywords: adverse effects; immune checkpoint inhibitors; ipilimumab; nivolumab; pembrolizumab; skin toxicity.
©2021 Apalla et al.
Conflict of interest statement
Competing interests: The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance.Cutan Ocul Toxicol. 2022 Mar;41(1):73-90. doi: 10.1080/15569527.2022.2034842. Epub 2022 Feb 20. Cutan Ocul Toxicol. 2022. PMID: 35107396 Review.
-
Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy.Am J Clin Dermatol. 2018 Jun;19(3):345-361. doi: 10.1007/s40257-017-0336-3. Am J Clin Dermatol. 2018. PMID: 29256113 Review.
-
Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors.Postepy Dermatol Alergol. 2019 Aug;36(4):382-391. doi: 10.5114/ada.2018.80272. Epub 2019 Aug 30. Postepy Dermatol Alergol. 2019. PMID: 31616210 Free PMC article. Review.
-
Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies.Curr Opin Oncol. 2016 Jul;28(4):254-63. doi: 10.1097/CCO.0000000000000290. Curr Opin Oncol. 2016. PMID: 27136138 Review.
-
Dermatologic immune-related adverse events: The toxicity spectrum and recommendations for management.Int J Womens Dermatol. 2021 Oct 23;7(5Part A):625-635. doi: 10.1016/j.ijwd.2021.10.005. eCollection 2021 Dec. Int J Womens Dermatol. 2021. PMID: 35005180 Free PMC article. Review.
Cited by
-
Narrative Review of Drug-Associated Nail Toxicities in Oncologic Patients.Dermatol Pract Concept. 2023 Jan 1;13(1):e2023064. doi: 10.5826/dpc.1301a64. Dermatol Pract Concept. 2023. PMID: 36892360 Free PMC article. Review.
-
Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article.Curr Oncol. 2022 Apr 18;29(4):2871-2886. doi: 10.3390/curroncol29040234. Curr Oncol. 2022. PMID: 35448208 Free PMC article. Review.
-
Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database.EClinicalMedicine. 2021 Jun 10;37:100951. doi: 10.1016/j.eclinm.2021.100951. eCollection 2021 Jul. EClinicalMedicine. 2021. PMID: 34386743 Free PMC article.
-
Morbilliform Eruptions: Differentiating Low-Risk Drug Eruptions, Severe Cutaneous Adverse Reactions, Viral Eruptions, and Acute Graft-Versus-Host Disease.Am J Clin Dermatol. 2025 May;26(3):379-393. doi: 10.1007/s40257-025-00924-0. Epub 2025 Jan 31. Am J Clin Dermatol. 2025. PMID: 39888589 Free PMC article. Review.
-
Cutaneous Side Effects of PD-1 Inhibitors: A Single-Center Retrospective Study.Int J Dermatol. 2025 Jun;64(6):1066-1078. doi: 10.1111/ijd.17683. Epub 2025 Feb 9. Int J Dermatol. 2025. PMID: 39925013 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
