miRNA in food simultaneously controls animal viral disease and human tumorigenesis
- PMID: 33614246
- PMCID: PMC7868940
- DOI: 10.1016/j.omtn.2021.01.011
miRNA in food simultaneously controls animal viral disease and human tumorigenesis
Abstract
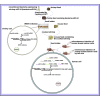
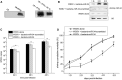
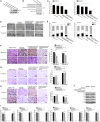
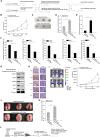
During virus infection in animals, the virus completes its life cycle in a host cell. A virus infection results in the metabolic deregulation of its host and leads to metabolic disorders, ultimately paving the way for cancer progression. Because metabolic disorders in virus infections occurring in animal are similar to metabolic disorders in human tumorigenesis, animal antiviral microRNAs (miRNAs), which maintain the metabolic homeostasis of animal cells, in essence, may have anti-tumor activity in humans. However, that issue has not been investigated. In this study, shrimp miR-34, a potential antiviral miRNA of shrimp against white spot syndrome virus (WSSV) infection, was identified. Overexpression of shrimp miR-34 in shrimp fed bacteria expressing miR-34 suppressed WSSV infection by targeting the viral wsv330 and wsv359 genes. Furthermore, the expression of shrimp miR-34 in mice fed miR-34-overexpressing shrimp suppressed breast cancer progression by targeting human CCND1, CDK6, CCNE2, E2F3, FOSL1, and MET genes. Therefore, our study suggests that the miRNAs in food could be an effective strategy for synchronously controlling viral diseases of economic animals and cancers in humans.
Keywords: breast cancer; food intake; shrimp miR-34; virus infection.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Shrimp miR-34 from Shrimp Stress Response to Virus Infection Suppresses Tumorigenesis of Breast Cancer.Mol Ther Nucleic Acids. 2017 Dec 15;9:387-398. doi: 10.1016/j.omtn.2017.10.016. Epub 2017 Oct 26. Mol Ther Nucleic Acids. 2017. PMID: 29246317 Free PMC article.
-
Shrimp Antiviral mja-miR-35 Targets CHI3L1 in Human M2 Macrophages and Suppresses Breast Cancer Metastasis.Front Immunol. 2018 Sep 12;9:2071. doi: 10.3389/fimmu.2018.02071. eCollection 2018. Front Immunol. 2018. PMID: 30258444 Free PMC article.
-
Two White Spot Syndrome Virus MicroRNAs Target the Dorsal Gene To Promote Virus Infection in Marsupenaeus japonicus Shrimp.J Virol. 2017 Mar 29;91(8):e02261-16. doi: 10.1128/JVI.02261-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28179524 Free PMC article.
-
Recent insights into host-pathogen interaction in white spot syndrome virus infected penaeid shrimp.J Fish Dis. 2015 Jul;38(7):599-612. doi: 10.1111/jfd.12279. Epub 2014 Jun 23. J Fish Dis. 2015. PMID: 24953507 Review.
-
A model for apoptotic interaction between white spot syndrome virus and shrimp.Fish Shellfish Immunol. 2013 Apr;34(4):1011-7. doi: 10.1016/j.fsi.2012.05.030. Epub 2012 Jun 7. Fish Shellfish Immunol. 2013. PMID: 22683516 Review.
Cited by
-
Live-cell imaging of microRNA expression with post-transcriptional feedback control.Mol Ther Nucleic Acids. 2021 Aug 26;26:547-556. doi: 10.1016/j.omtn.2021.08.018. eCollection 2021 Dec 3. Mol Ther Nucleic Acids. 2021. PMID: 34631284 Free PMC article.
-
Antiviral Shrimp lncRNA06 Possesses Anti-Tumor Activity by Inducing Apoptosis of Human Gastric Cancer Stem Cells in a Cross-Species Manner.Mar Drugs. 2024 May 15;22(5):221. doi: 10.3390/md22050221. Mar Drugs. 2024. PMID: 38786611 Free PMC article.
-
MicroRNA Regulation in Infectious Diseases and Its Potential as a Biosensor in Future Aquaculture Industry: A Review.Molecules. 2023 May 26;28(11):4357. doi: 10.3390/molecules28114357. Molecules. 2023. PMID: 37298833 Free PMC article. Review.
-
Non-coding RNA-related antitumor mechanisms of marine-derived agents.Front Pharmacol. 2022 Dec 1;13:1053556. doi: 10.3389/fphar.2022.1053556. eCollection 2022. Front Pharmacol. 2022. PMID: 36532760 Free PMC article. Review.
-
MicroRNAs and the Mediterranean diet: a nutri-omics perspective for lung cancer.J Transl Med. 2024 Jul 7;22(1):632. doi: 10.1186/s12967-024-05454-7. J Transl Med. 2024. PMID: 38972997 Free PMC article. Review.
References
-
- Kong T., Ren X., Lin S., Li S., Gong Y. Elucidation of metabolic responses in mud crab Scylla paramamosain challenged to WSSV infection by integration of metabolomics and transcriptomics. Dev. Comp. Immunol. 2020;113:103799. - PubMed
-
- Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008;6:541–552. - PubMed
-
- Diamond D.L., Syder A.J., Jacobs J.M., Sorensen C.M., Walters K.A., Proll S.C., McDermott J.E., Gritsenko M.A., Zhang Q., Zhao R. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6:e1000719. - PMC - PubMed
-
- Arnold P.A., Johnson K.N., White C.R. Physiological and metabolic consequences of viral infection in Drosophila melanogaster. J. Exp. Biol. 2013;216:3350–3357. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

