A novel approach to minimize the false negative COVID-19 diagnosis by inclusion of specific cell markers and multiple sample collection
- PMID: 33614422
- PMCID: PMC7881297
- DOI: 10.1016/j.mex.2021.101270
A novel approach to minimize the false negative COVID-19 diagnosis by inclusion of specific cell markers and multiple sample collection
Abstract
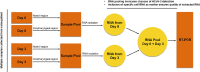
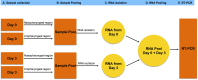
The SARS-CoV-2 pandemic has caused unpredictable mortality and economic losses globally. With no approved drug for the treatment, the accurate diagnosis of COVID-19 becomes essential. RNA based test takes several hours and require extensive human intervention for RNA extraction and RT-PCR, but it is preferred over the antibody-based detection as the latter does not detect the early stage infections. The RT-PCR being a gold standard of COVID-19 diagnosis offers highly standardized detection of the SARS-CoV-2 RNA, still vulnerable for false-negative diagnosis due to absence of infected cells in the sample or inaccurate RNA extraction. Hence there is a need to develop alternative protocols and methods for the accurate COVID-19 diagnosis. Here we propose two additional steps in RT-PCR based COVID-19 diagnosis to minimize false-negative detection. The first step involves collection of four samples from an individual. Each sample should be collected from nasopharyngeal and oropharyngeal regions on day 01, mixed together followed by RNA extraction and then repeating the same exercise on day 03. The RNA extracted on day 01 and day 03 must be pooled together to be used in the RT-PCR. Second, we propose the inclusion of the control marker genes specific to nasal goblet cell, type-II pneumocyte and absorptive enterocytes to ensure the specificity of the RNA source. Overall, these additional steps in the proposed method would increase the chances of SARS-CoV-2 detection in the infected population and would limit the false-negative diagnosis of COVID-19 and hence the spread of this disease.•RT-PCR based COVID-19 diagnosis is vulnerable to the false-negative results due to inaccurate sample isolation or RNA extraction.•RNA pool of multiple samples from an individual improves the chances of detection of SARS-CoV-2 by RT-PCR.•Inclusion of specific marker genes would ensure the right RNA source from the desired cell.
Keywords: COVID-19; COVID-19, Coronavirus disease 2019; RT-PCR; RT-PCR, Reverse Transcriptase PCR; RdRp, RNA dependent RNA polymerase; SARS-CoV-2; SARS-CoV-2, Human Coronavirus-2.
© 2021 Published by Elsevier B.V.
Conflict of interest statement
Author has no conflict of interest in regards to the information shared in the manuscript.
Figures
Similar articles
-
Strategies to Overcome Erroneous Outcomes in Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Testing: Insights From the COVID-19 Pandemic.Cureus. 2024 Nov 3;16(11):e72954. doi: 10.7759/cureus.72954. eCollection 2024 Nov. Cureus. 2024. PMID: 39498425 Free PMC article. Review.
-
Value of swab types and collection time on SARS-COV-2 detection using RT-PCR assay.J Virol Methods. 2020 Dec;286:113974. doi: 10.1016/j.jviromet.2020.113974. Epub 2020 Sep 16. J Virol Methods. 2020. PMID: 32949663 Free PMC article.
-
Positive control synthesis method for COVID-19 diagnosis by one-step real-time RT-PCR.Clin Chim Acta. 2020 Dec;511:149-153. doi: 10.1016/j.cca.2020.10.001. Epub 2020 Oct 12. Clin Chim Acta. 2020. PMID: 33058837 Free PMC article.
-
[Evaluation of a Visually-Read Rapid Antigen Test Kit (SGA V-Chek) for Detection of SARS-CoV-2 Virus].Mikrobiyol Bul. 2021 Jul;55(3):461-464. doi: 10.5578/mb.20219815. Mikrobiyol Bul. 2021. PMID: 34416811 Turkish.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
Cited by
-
Improved DNA extraction on bamboo paper and cotton is tightly correlated with their crystallinity and hygroscopicity.PLoS One. 2022 Nov 7;17(11):e0277138. doi: 10.1371/journal.pone.0277138. eCollection 2022. PLoS One. 2022. PMID: 36342943 Free PMC article.
-
Importance of Adequate qPCR Controls in Infection Control.Diagnostics (Basel). 2021 Dec 16;11(12):2373. doi: 10.3390/diagnostics11122373. Diagnostics (Basel). 2021. PMID: 34943608 Free PMC article.
-
Persistence of SARS-CoV-2 in saliva: Implications for late-stage diagnosis and infectious duration.PLoS One. 2023 Mar 16;18(3):e0282708. doi: 10.1371/journal.pone.0282708. eCollection 2023. PLoS One. 2023. PMID: 36928472 Free PMC article.
-
Strategies to Overcome Erroneous Outcomes in Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Testing: Insights From the COVID-19 Pandemic.Cureus. 2024 Nov 3;16(11):e72954. doi: 10.7759/cureus.72954. eCollection 2024 Nov. Cureus. 2024. PMID: 39498425 Free PMC article. Review.
References
-
- Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A., Ferdosian F., Bahrami R. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr. Pathol. 2020;39(3):246–250. JunPMID: 32238084. - PMC - PubMed
-
- Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., Bonazzetti C., Covizzi A., Schiuma M., Passerini M., Piscaglia M., Coen M., Gubertini G., Rizzardini G., Cogliati C., Brambilla A.M., Colombo R., Castelli A., Rech R., Riva A., Torre A., Meroni L., Rusconi S., Antinori S., Galli M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol. Res. 2020;158 AugPMID: 32446978. - PMC - PubMed
-
- Kumar P., Sah A.K., Tripathi G., Kashyap A., Tripathi A., Rao R., Mishra P.C., Mallick K., Husain A., Kashyap M.K. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol. Cell Biochem. 2020;7:1–22. OctPMID: 33029696. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

