The MRI-Visible Nanocomposite Facilitates the Delivery and Tracking of siRNA Loaded DC Vaccine in the Breast Cancer Model
- PMID: 33614503
- PMCID: PMC7892972
- DOI: 10.3389/fonc.2020.621642
The MRI-Visible Nanocomposite Facilitates the Delivery and Tracking of siRNA Loaded DC Vaccine in the Breast Cancer Model
Abstract
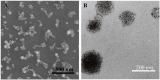
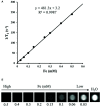
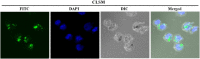
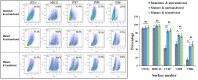
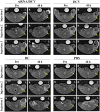
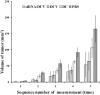
Dendritic cell (DC) vaccines have recently been developed for the treatment of various cancers but often do not function as well as expected, primarily due to the highly complex in vivo immune environment. This proof-of-principle study aimed to test the feasibility of modulating the in vivo behaviors of DC vaccines (DCVs) by introducing siRNA-laden magnetic resonance (MR) imaging nanovectors into cells, while providing visible information on their homing to lymph nodes. The N-alkyl-PEI2k-LAC/SPIO nanocomposites were prepared and characterized, showing favorable properties of siRNA transfection and MRI labeling efficiency in DCs. Cell viability assays revealed no observable effects on the survival and phenotype of DCs if the concentration of the complex was within 8 μg Fe/ml. An orthotopic mouse model of breast cancer was developed. The DCVs transfected with IDO siRNA contained nanocomposites were adoptively transferred to start the treatment. MR imaging clearly visualized the homing of DCVs into lymph nodes. At the end of the treatment, DCVs presented significantly better tumor suppression than DCs or PBS (P < 0.05). Generally, the N-alkyl-PEI2k-LAC/SPIO nanocomposites represent a highly efficient MR imaging platform for siRNA transfection that is potentially useful for in vivo tracking of vaccine cells.
Keywords: anticancer immunotherapy; dendritic cell; gene transfection; magnetic resonance imaging; nanocomposite.
Copyright © 2021 Wu, Zhu, Jin, Ai and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Superparamagnetic MRI probes for in vivo tracking of dendritic cell migration with a clinical 3 T scanner.Biomaterials. 2015 Jul;58:63-71. doi: 10.1016/j.biomaterials.2015.04.016. Epub 2015 May 4. Biomaterials. 2015. PMID: 25941783
-
In vivo tracking and immunological properties of pulsed porcine monocyte-derived dendritic cells.Mol Immunol. 2015 Feb;63(2):343-54. doi: 10.1016/j.molimm.2014.08.006. Epub 2014 Oct 1. Mol Immunol. 2015. PMID: 25282042
-
Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy.Theranostics. 2016 Sep 2;6(11):2000-2014. doi: 10.7150/thno.15102. eCollection 2016. Theranostics. 2016. PMID: 27698936 Free PMC article.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Migration of dendritic cells to the lymph nodes and its enhancement to drive anti-tumor responses.Crit Rev Oncol Hematol. 2016 Nov;107:100-110. doi: 10.1016/j.critrevonc.2016.09.002. Epub 2016 Sep 9. Crit Rev Oncol Hematol. 2016. PMID: 27823637 Review.
Cited by
-
Cancer Immunotherapy and Medical Imaging Research Trends from 2003 to 2023: A Bibliometric Analysis.J Multidiscip Healthc. 2024 May 6;17:2105-2120. doi: 10.2147/JMDH.S457367. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38736544 Free PMC article. Review.
-
Biomaterials' enhancement of immunotherapy for breast cancer by targeting functional cells in the tumor micro-environment.Front Immunol. 2024 Nov 12;15:1492323. doi: 10.3389/fimmu.2024.1492323. eCollection 2024. Front Immunol. 2024. PMID: 39600709 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

